Nitric acid (also known
as aqua fortis and
spirit of niter).
Molecular formula : HNO3
(O= 76,17 %, N= 22,23 %, H= 1,6 %)
Molar mass = 63,0128 ± 0,0012 g·mol-1
It is a highly corrosive strong mineral acid. Nitric acid is the
primary reagent used for nitration - the addition of a nitro group,
typically to an organic molecule. While some resulting nitro
compounds are shock- and thermally-sensitive explosives, a few are
stable enough to be used in munitions and demolition, while others
are still more stable and used as pigments in inks and dyes. Nitric
acid is also commonly used as a strong oxidizing agent.
Nitric acid is a component of acid rain,
which is formed by hydration of NO2 nitrogen dioxide, a
major air pollutant:
3 NO2 + H2O >>> 2
HNO3 + NO
Normally, the nitric oxide produced by the reaction is reoxidized
by the oxygen in air to produce additional nitrogen dioxide.
Preparation.
Production of nitric acid is via the Ostwald process,
named after German chemist Wilhelm Ostwald. In this process,
anhydrous ammonia is oxidized to nitric oxide, in the presence of
platinum or rhodium gauze catalyst at a high temperature of about
226.9 °C (500 K, 440.3 °F) and a pressure of 9 bar:
4 NH3 (g) + 5 O2 (g) >>> 4 NO
(g) + 6 H2O (g) (DH = -905.2
kJ)
Nitric oxide is then reacted with oxygen in air to form nitrogen
dioxide.
2 NO (g) + O2 (g) >>> 2 NO2
(g) (DH = -114 kJ/mol)
This is subsequently absorbed in water to form nitric acid and
nitric oxide.
3 NO2 (g) + H2O (L) >>> 2
HNO3 (aq) + NO (g) (DH = -117
kJ/mol)
The nitric oxide is cycled back for reoxidation. Alternatively, if
the last step is carried out in air:
4 NO2 (g) + O2 (g) + 2 H2O
(l) >>> 4 HNO3 (aq)
The aqueous HNO3 obtained can be concentrated by
distillation up to about 68% by mass. Further concentration to 98%
can be achieved by dehydration with concentrated H2SO4.
By using ammonia derived from the Haber process, the final product
can be produced from nitrogen, hydrogen, and oxygen which are derived
from air and natural gas as the sole feedstocks.
The annual global production of nitric acid (by various processes) is
of the order of 60 million tons.
Physical and chemical properties :
Commercially available nitric acid is an azeotrope with water at
a concentration of 68% HNO3, which is the ordinary
concentrated nitric acid of commerce. This solution has a boiling
temperature of 120.5 °C at 1 atm.
Two solid hydrates are known; the monohydrate
(HNO3·H2O) and the trihydrate
(HNO3·3H2O).
Nitric acid of commercial interest usually consists of the maximum
boiling azeotrope of nitric acid and water, which is approximately
68% HNO3, (approx. 15 molar). This
is considered concentrated or technical grade, while reagent grades
are specified at 70% HNO3. The density of concentrated
nitric acid is 1.42 g/mL. An older density scale is occasionally
seen, with concentrated nitric acid specified as 42°
Baumé.
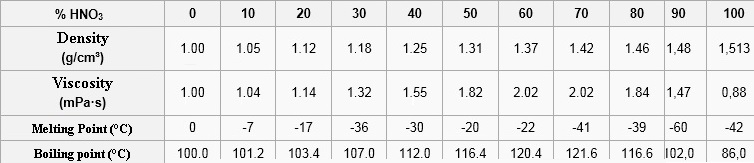
Main properties (at 20 ° C and 1.013
bar) :
Nitric acid is normally considered to be a strong acid at ambient
temperatures. There is some disagreement over the value of the acid
dissociation constant, though the pKa value is usually reported as
less than -1. This means that the nitric acid in solution is fully
dissociated except in extremely acidic solutions. The pKa value rises
to 1 at a temperature of 250 °C.
Mixed with hydrochloric acid, it forms "aqua
regia", one of the few reagents capable of dissolving gold and
platinum.
Commercial solutions.
Commercial grade nitric acid solutions are usually between 52%
and 68% nitric acid.
When the solution contains more than 86% HNO3, it is referred to as
fuming nitric acid. Depending on the amount of nitrogen dioxide
present, fuming nitric acid is further characterized as white fuming
nitric acid or red fuming nitric acid, at concentrations above
95%.
A formulation for the water
treatment.
Reaction mechanism of calcium bicarbonates (simplified
equations):
.....................
...................HNO3
+.....Ca[HCO3]2
.
......>>>.... .....
2CO2 ....+
H2O. +
Ca[NO3]2
.................(63)............
(162 or
10°f)
...........................(44x2)
.......
With 1 mg as HNO3 added, there has (10/63) = 0.15 °f for
lower TAC (Alkalinity), and 1.396 mg as free CO2 and 1.58 mg/L as
NO3 formed.
Storage.
Takes place in containers inxoydables steel or amuminium for over
80% levels.
Glass containers for small volumes.
Non-compatible materials: nichel, copper.
Uses.
The main industrial use of nitric acid is for the
production of fertilizers. Nitric acid is neutralized with ammonia to
give ammonium nitrate. This application consumes 75–80% of the
26M tons produced annually (1987). The other main applications are
for the production of explosives, nylon precursors, and specialty
organic compounds.
- Wastewater and industrial treatment (as dilute aqueous
solutions).
- In a low concentration (approximately
10%), nitric acid is often used to artificially age pine
and maple. The color produced is a grey-gold very much like very
old wax or oil finished wood (wood finishing).
- The corrosive effects of nitric acid are exploited for a
number of specialty applications, such as pickling stainless
steel. A solution of nitric acid, water and alcohol, Nital, is
used for etching of metals to reveal the microstructure. ISO 14104
is one of the standards detailing this well known procedure.
- Commercially available aqueous blends of 5–30% nitric
acid and 15–40% phosphoric acid are commonly used for
cleaning food and dairy equipment primarily to remove precipitated
calcium and magnesium compounds (either deposited from the process
stream or resulting from the use of hard water during production
and cleaning). The phosphoric acid content helps to passivate
ferrous alloys against corrosion by the dilute nitric acid.
- Nitric acid can be used as a spot test for alkaloids like LSD,
giving a variety of colours depending on the alkaloid.
- In organic synthesis, industrial and otherwise, the nitro
group is a versatile functionality. Most derivatives of aniline
are prepared via nitration of aromatic compounds followed by
reduction. Nitrations entail combining nitric and sulfuric acids
to generate the nitronium ion, which electrophilically reacts with
aromatic compounds such as benzene. Many explosives, e.g. TNT, are
prepared in this way.
- Nitric acid has been used in various forms as the oxidizer in
liquid-fueled rockets.
These forms include red fuming nitric acid, white fuming nitric
acid, mixtures with sulfuric acid, and these forms with HF
inhibitor. IRFNA (inhibited red fuming nitric acid) was one of 3
liquid fuel components for the US BOMARC missile.
Health-wise.
Nitric acid is a strong acid and a powerful oxidizing agent. The
major hazard posed by it is chemical burns as it carries out acid
hydrolysis with proteins (amide) and fats (ester) which consequently
decomposes living tissue (e.g. skin and flesh). Concentrated nitric
acid stains human skin yellow due to its reaction with the keratin.
These yellow stains turn orange when neutralized. Systemic effects
are unlikely, however, and the substance is not considered a
carcinogen or mutagen.
The standard first aid treatment for acid spills on the skin is, as
for other corrosive agents, irrigation with large quantities of
water. Washing is continued for at least ten to fifteen minutes to
cool the tissue surrounding the acid burn and to prevent secondary
damage. Contaminated clothing is removed immediately and the
underlying skin washed thoroughly.
Being a strong oxidizing agent, reactions of nitric acid with
compounds such as cyanides, carbides, metallic powders can be
explosive and those with many organic compounds, such as turpentine,
are violent and hypergolic (i.e. self-igniting). Hence, it should be
stored away from bases and organics.
Toxicological profile (French) - Acide nitrique
(FT
9) - Institut national de recherche et de sécurité
(INRS).
Sources : personal and Wikipedia,
the free encyclopedia.
 (use your browser)
(use your browser)
 (use your browser)
(use your browser)