Hydrochloric acid
(hydrogen chloride).
Molecular formula : HCL
(Cl = 97,23 %, H = 2,76 %)
Molar mass = 36,461 ± 0,002 g·mol-1
Formerly called "spirit of salt" or muriatic acid. This acid is a
colorless liquid with a pungent odor. Concentrate can have a pH less
than 1, and very corrosive.
Pure hydrochloric acid does not exist on Earth in natural state.
However, this is a chemical species that has been frequently used in
history from the beginnings of chemistry (using salts in presence of
sulfuric acid, Jabir Ibn Hayyan Persian alchemist discovered
hydrochloric acid from sodium chloride, about the year 800).
Note : HCl is the major component of gastric acid. It is present in
the stomach where it helps the digestion of food.
Preparation.
Reacting the Cl2 chlorine
gas which reacts with slight excess of H2 dihydrogen,
to produce hydrogen chloride gas HCl (the reaction takes place in a
burner, to 2000 ° C).
Cl2 + H2 >>> 2 HCl gas
Then the latter is absorbed in water:
HCL + water >>> HCl aqueous
Other methods based on same principle of "burning" of carbon
(coke) and sulfur dioxide in presence of chlorine and water
vapor:
C + 2Cl2 + 2H2O >>> 4 HCl + CO2
SO2 + Cl2 + 2H2O + >>> 2 HCl + H2SO4
Reaction between H2SO4 (sulfuric acid)
and chloride Cl:
(2-step reaction with salt containing chloride [mCl])
H2SO4 + mCl >>> mHSO4 + HCl
mHSO4 + mCl >>> m2SO4 + HCl
Note:
The first reaction takes place at 150 to 300° C, while second
stage requires a temperature of about 550 to 600° C and excess
salt.
The Mannheim and Berlin process using this synthetic route.
Main features:
The physical properties of hydrochloric acid, such as boiling
point or melting, depend on the concentration (or
molar aqueous HCl solution). They since the physical
properties of water varies from 0% HCl to those of fuming
hydrochloric acid for higher fractions of 40% HCl.
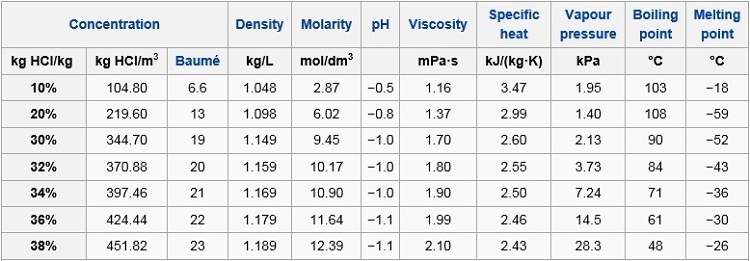
The reference temperature and pressure for the above table are 20
°C and 1 atmosphere (101.325 kPa).
Technical solutions :
- 10 to 12% (density: about 1048 kg/m3)
- 30-34% (density: 1149 à 1169 kg/m3)
In water treatment, it is used in the injection water as dilute
commercial solutions.
A formulation for the water treatment.
Reaction mechanism of calcium bicarbonates (simplified
equations):
..........................2 HCl +
Ca[HCO3]2 ...>>> ....2
CO2 ...+ ...2H2O + CaCl2
.......................73.......162 ou 10°.............
.(2x44=88)
........................111
With 1 mg as HCl added, there has (10/73) = 0.137°F for lower
TAC, and 1.205 mg as free CO2 and (71/73)=
0.97 mg/L as Cl chloride formed.
Storage.
Tightly closed and dry in a well ventilated area.
For current storage: no metal containers.
Uses.
- water treatment (drinking, waste and industrial) and treatment
pools
- household cleaner
- used in many industrial processes, including:
- construction
- leather processing
- fertilizer manufacturing
- oil production
- pickling and descaling metal
- producing inorganic compounds
- regeneration of ion exchange resins
- manufacture of various chlorides and metallic salts
- production of food ingredients and food additives
Health-wise.
- Inhalation: vapors may be fatal
- Ingestion: toxic, sometimes fatal
- Skin: May cause severe injury
- Eyes very dangerous
Sources : personal and Wikipedia,
the free encyclopedia.
 (use your browser)
(use your browser)

 (use your browser)
(use your browser)