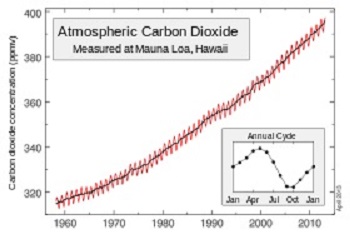
General:
Carbon dioxide is the result of combination of two elements: a
carbon atom C and two oxygen atoms O [chemical formula =
CO2].
Molar mass 44.01 g mol-1.
It is produced by different processes : in particular during aerobic
fermentation or combustion of organic compounds, and when the
respiration of living beings, and plants. For the latter,
photosynthesis trap much more CO2 than respiration
produces.
It is found at low levels in the atmospheric air or is absorbed by
plants, which in turn produce oxygen. There is present in an amount
approximately equal to 0.04% by volume for the current decade
(WMO) or 400 ppmv (parts per
million by volume) or 591 parts per million by mass. But it
increases rapidly from about 1.43 ppmv / year by human activities
consumption of fossil fuels: coal, oil, gas.

VALUES OF THE PARTIAL PRESSURE CO2:
In the atmosphere, it was in the late 1970s 2.10-4 atm,
and twenty years later, due to the evolution of human activities is
average 3.4 10-4 atm and currently 3.89 10-4
atm (0.394 hPa). It is a greenhouse gas (GHG
see
here on the Hydro-Land french site)
whose relative contribution to this phenomenon would be 49% (methane
CH4 18%).
Properties.
CO2 gas has a slightly irritating odor, is colorless
and heavier than air.
In aqueous solution, it forms carbonic acid
H2CO3 (H2O + CO2
<==> H2CO3) is too unstable to be
isolated easily.
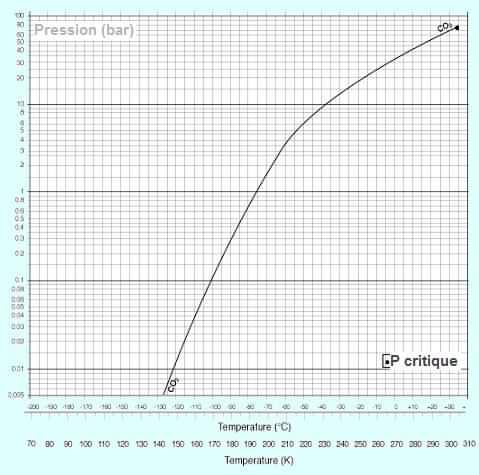
The phase diagram of carbon dioxide (CO2, or
carbonic anhydride) below shows that, unlike water, the solid
curve (dry ice) and liquid
(liquid CO2) has a positive slope: ice CO2 is
denser than the liquefied gas.
Note: Density of the liquid phase (at -20 ° C and 19.7 bar):
1032 kg/m3 - Density of solid: 1562 kg/m3.
Only gas present in four forms: solid, liquid, gas and supercritical.
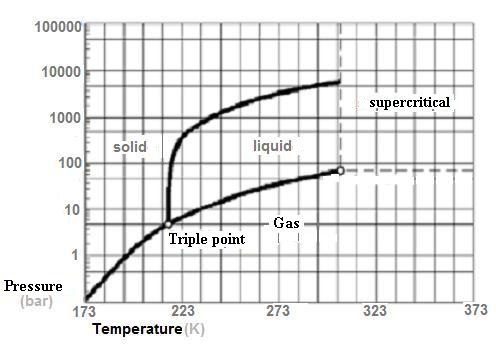
Furthermore, the triple point (temperature = 56.6° C (216.5
K) and pressure: 5180 hPa [5.18 bar]) is greater than the
atmospheric "normal" pressure of 1013.25 hPa (1.013 bar ) and
therefore the liquid CO2 can not exist at this pressure
(it can not melt or boil at this pressure),
but it can sublimate has all lower pressures.
The Critical Point is located Pc = 7.375 MPa (73.75 bar) and Tc =
30.95 ° C [304.1 K].
The solid CO2 is called dry ice (because it does not
melt, it sublimates). Note that at 1 atm, the temperature of
the sublimation of dry ice is -78.2 ° C (194.95 K). The CO2 is
normally carried in the liquid state into steel cylinders under
pressure (- 20 ° C and 20 bar).

Gas solubility in water (101.3 kPa and 0° C): 1.7 vol / vol.
> Solubility in grams per liter, depending on the temperature
(under NTP > 20°C (293.15 K,
68°F) and 1 atm (101.325 kN/m2, 101.325
kPa):
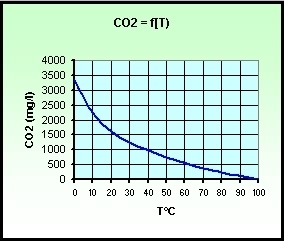
Note - equilibrium,
is governed by Henry's constant whose value is (101.3 kPa and 25°C):
with pCO2 = partial pressure of CO2 and
[H2CO3] =
activity.
- This constant varies with temperature according to the empirical
expression:
with t, temperature in ° Celsius.
- Note that the activity [H2CO3] represents the real molecule H2CO3 (carbonic acid) and dissolved molecular CO2 present in the form of microbubbles.
Thus for example, in a water surface at 25 ° C, we have:
about 0.53 g of free CO2 per kg pure water, or 0.53 mg / liter.
Some features:
- Inert , colorless and non-flammable.
- Equivalent gas / liquid (1013 hPa and 15°C [ kg solid ] ) : 845 vol / vol
- Latent heat of vaporization of liquid CO2 (1013 hPa at boiling point ) : 571.08 kJ kg-1
- Vapor pressure of liquid CO2 ( 20°C): 5.85 MPa (58.5 bar)
- Triple point temperature = -56.6 ° C - Pressure: 5180 hPa (5.18 bar)
- Latent heat of fusion of dry ice (1013 hPa , at triple point ) : 196.104 kJ kg-1
- Critical point : Temperature: 31°C ( 304 K) and critical pressure: 7.38 MPa (73.8 bar)
- Density at the critical point (liquid / gas): 464 kg/m3
- Density of gas at 1013 hPa ( 1 atm ) : 2.814 kg m3 -1 at -78°C and 1.87 kg m3 -1 at 15 ° C
- Specific heat of gas at constant pressure (Cp ) (1013 hPa and 25°C ) : 37 J / ( mole.K )
- Specific heat of gas at constant volume ( Cv) (1013 hPa and 25°C): 28 J / ( mole.K )
- Viscosity of gas (1013 hPa and 0 ° C ) : 0.0001372 Poise
- Average concentration of CO2 in the air (mid 2010): 0.0389 % by volume (or 0.0585 % by weight) .
and solid or Dry Ice (also known as Carbonice). The dry ice sublimates, leaving no residue and produced very quickly a large amount of cold ( -78.5 ° C; -109.3 °F; 194.7 K), the man has quickly found him many uses:
Like water, beyond its critical point (see above), carbon dioxide
enters a phase called "supercritical".
The gas liquid equilibrium curve is interrupted at the critical
point, ensuring a supercritical phase in the continuum of physical
and chemical properties without phase change. It is as dense as a
liquid phase, but providing transport properties (viscosity,
diffusion) near a gas.
The supercritical carbon dioxide is used as a "green" solvent, the
extracts are free of traces of solvent:
Industrial applications.
Pulp and Paper .
Carbon dioxide finely regulate the pH of the recycled
mechanical or chemical after alkaline pulp bleaching. It can also be
used in the neutralization of "tall oil" and to improve the operation
of paper machines.
Chemical industry.
Carbon dioxide is used in chemical synthesis or for the
temperature control of reactors. CO2 is also used to neutralize
alkaline effluents.
It is operated under supercritical conditions or to perform
operations purifications dyes polymer or vegetable or animal fibers.
Pharmaceutical industry.
Carbon dioxide is used for inerting, chemical synthesis in
supercritical extraction (SFE), neutralization (pH) of wastewater or
transportation of goods at low temperature (-78°C or
-108°F).
Food and Drink .
CO2 is used in the food sector in these main areas:
Health.
The CO2 atmosphere produces a near physiological conditions
during handling of artificial organs.
It is used in admixture with air or oxygen for respiration boost.
It is also used for surgical dilation intraabdominal
insufflation.
Metal industry
Carbon dioxide is commonly used for environmental protection:
Laboratories and analyzes.
CO2 is used in the mobile phase extraction processes or
supercritical fluid chromatography.
Electronic.
Carbon dioxide is commonly used in the wastewater treatment or as
a cooling medium in the climatic test of electronic components.
It can be used to increase the conductivity of the ultrapure water or
for the abrasive cleaning of parts in the form of dry ice in the
cleaning process own photoresists supercritical CO2 in order to avoid
the use of organic solvents.
Environment.
The injection of carbon dioxide makes pH control of the aqueous
effluents. CO2 is a great alternative to control pH with sulfuric
acid.
Other industries.
Carbon dioxide extinguisher.
Control and regulation of the pH of the waste water, swimming pools,
etc.
MODE OF PROCUREMENT:
Carbon dioxide can be distributed:
Major risks:
Link
(Canadian) on the effects of CO2 for health
MATERIAL SAFETY DATA SHEET (French INRS): pdf file
to download possibly (71 kb).
 (use your browser)
(use your browser)