Sodium carbonate (also
known as washing soda,
soda ash and soda
crystals).
Chemical formule : Na2CO3
(O = 45,29 %, Na = 43,38 %, C = 11,33 %)
Molar mass = 105,9884 ± 0,0017
g·mol-1
Hygroscopic white powder (anhydrous), it is the sodium salt of
carbonic acid [CO3, H2O]; in everyday language, also known as
washing soda, because of its sodium content and its usually
crystalline form. However, this should not be confused with salt
Caustic soda NaOH, or with
sodium bicarbonate
NaHCO3.
Sodium carbonate crystallizes from water to form three different
hydrates:
Production (natural) :
Sodium carbonate is soluble in water, and can occur naturally in
arid regions, especially in mineral deposits (evaporites) formed when
seasonal lakes evaporate. Deposits of the mineral natron* have
been mined from dry lake bottoms in Egypt since ancient times, when
natron was used in the preparation of mummies and in the early
manufacture of glass. The anhydrous mineral form of sodium carbonate
is quite rare and called natrite.
*evaporite rock containing sodium carbonate decahydrate
(Na2CO3·10H2O) and sodium
bicarbonate (NaHCO3).
Formerly, it is also produced from the ashes of seaweed (kelp or
seaweed in Brittany) or plants (Salicornia in the
Mediterranean).
Synthetic product.
Solvay process (1870)
which produces sodium carbonate from salt and chalk supplanted
Leblanc process (developed in 1789) as cleaner and less
expensive.
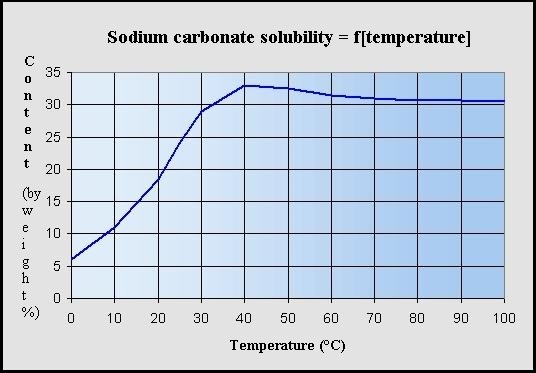
Main Features :
Maximum solubility in water (soft) as a function of the
temperature (Na2CO3 % by weight ) :

Solutions at 50g/L :
A formulation for water treatment.
Reaction mechanism of lime with aggressive CO2 (simplified
equations):
Na2CO3 +
CO2 + H2O >>> 2 NaHCO3
....106.........44
.....................2x84 or[100
mg/L as CaCO3 (10°F<TAC)]
Thus, to neutralize 1 mg of CO2, it is necessary to use
(106/44)= 2.41 mg de
Na2CO3, which form (168/44)=3.82 mg of sodium
bicarbonate NaHCO3, and therefore also the neutralization
of 1 mg /L to form CO2 (10/44) = 0.2273
°F/l as TAC (i.e.2.273 mg CaCO3/L of Alkalinity).
Note: if the calculated dose is a reagent for 100% purity, it is
necessary to correct this dose to entering the purity of the
commercial product.
Standardization.
Standard products used for the production of drinking water:
French
Official Bulletin - Sodium carbonate : NF EN 897.
Effects on the environment.
Sodium carbonate is not toxic to the environment. But it can be
irritating to the skin and so it is best handled with gloves.
Uses.
Sources : personal and Wikipedia, the free encyclopedia.
 (use your browser)
(use your browser)