Phosphoric acid (also
known as orthophosphoric acid or
phosphoric
[V] acid)
Molecular formula :
H3PO4
(O = 65,3 %, P = 31,64%, H = 3,06 %)
Molar mass = 97,9952 ± 0,0014 g·mol-1
Phosphoric acid is a mineral (inorganic) acid
. Orthophosphoric acid molecules can combine with themselves to form
a variety of compounds which are also referred to as phosphoric
acids, but in a more general way. The term phosphoric acid can also
refer to a chemical or reagent consisting of phosphoric acids, such
as pyrophosphoric acid or triphosphoric acid, but usually
orthophosphoric acid.
The conjugate base of phosphoric acid is the dihydrogen phosphate
ion, H2PO4-, which in turn has a
conjugate base of hydrogen phosphate, HPO42-,
which has a conjugate base of phosphate,
PO43-.
The most common source of phosphoric acid is an 85% aqueous solution;
such solutions are colourless, odourless, and non-volatile. Rather
viscous, syrupy liquids, but still pourable. Because it is a
concentrated acid, an 85% solution can be corrosive, although
nontoxic when diluted. Because of the high percentage of phosphoric
acid in this reagent, at least some of the orthophosphoric acid is
condensed into polyphosphoric acids. For the sake of labeling and
simplicity, the 85% represents H3PO4 as if it
were all orthophosphoric acid. Dilute aqueous solutions of phosphoric
acid exist in the ortho- form.
Preparation.
Phosphoric acid can be prepared by three routes :
- the wet process,
- which includes two sub-methods,
- the thermal process.
The more expensive thermal process produces a purer product that
is used for applications in the food industry. The wet process
dominates in the commercial sector.
Wet process phosphoric acid is prepared by adding sulfuric
acid H2SO4 to tricalcium phosphate rock
Ca5(PO4)3F, typically found in nature as
apatite.
The reaction is:
Ca5(PO4)3X + 5
H2SO4 + 10 H2O >>> 3
H3PO4 + 5 CaSO4·2
H2O + HX
where X may include OH, F, Cl, and Br.
Digestion of the phosphate ore using sulfuric acid yields the
insoluble calcium sulfate (gypsum, CaSO4.2H2O),
which is filtered and removed as phosphogypsum or hemihydrate
CaSO4 .1/2 H2O. Wet-process acid can be further
purified by removing fluorine to produce animal-grade phosphoric
acid, or by solvent extraction and arsenic removal to produce
food-grade phosphoric acid.
The nitrophosphate process is similar to the wet process
except that it uses nitric acid in place of sulfuric acid. The
advantage to this route is that the coproduct, calcium nitrate is
also a plant fertilizer. This method is rarely employed.
Thermal process.
Very pure phosphoric acid is obtained by burning elemental
phosphorus to produce phosphorus pentoxide
P4O10 (empirical formula,
P2O5), which is subsequently dissolved in dilute phosphoric
acid. This route produces a very pure phosphoric acid, since most
impurities present in the rock have been removed when extracting
phosphorus from the rock in a furnace. The end result is food-grade,
thermal phosphoric acid; however, for critical applications,
additional processing to remove arsenic compounds may be needed.
Elemental phosphorus is produced by an electric furnace. At a high
temperature, a mixture of phosphate ore, silica and carbonaceous
material (coke, coal etc...) produces calcium silicate, phosphorus
gas P and carbon monoxide CO. The P and CO off-gases from this
reaction are cooled under water to isolate solid phosphorus.
Alternatively, the P and CO off-gases can be burned with air to
produce phosphorus pentoxide and carbon dioxide CO2.
Main properties.
At room temperature, phosphoric acid is a crystalline solid
density 1.834, melting point at 42.35 °C > colorless viscous
liquid.
Pur is a hygroscopic solid (deliquescent). The pure acid is not
commercially available.
|
|
Product
|
|
Density
|
1.885 g/mL (liquid)
1.685 g/mL (85% solution)
|
|
Melting point
|
42.35 °C (108.23 °F)
(anhydrous) 29.32 °C (84.78
°F)
(hemihydrate)
|
|
Boiling point (decomposition)
|
158 °C (316 °F; 431
K)
|
|
Solubility
|
392.2 g/100 mL (-16.3 °C)
369.5 g/100 mL (0.5 °C)
5.48 g/mL (20 °C)
miscible (42.3 °C)
|
|
Viscosity
|
2.4–9.4 cP (85% aq. soln.)
147 cP (100%)
|
The oxidation state of phosphorus (P) in ortho- and other phosphoric
acids is +5; the oxidation state of all the oxygen atoms (O) is -2
and all the hydrogen atoms (H) is +1. Triprotic means that an
orthophosphoric acid molecule can dissociate up to three times,
giving up an H+ each time, which typically combines with a
water molecule, H2O, as shown in these reactions:
- H3PO4 (s) + H2O(l)
>>> H2PO4-(aq) +
H3O+(aq), Ka1=
7,25×10-3, pKa1 =
2,12
- H2PO4-(aq) +
H2O(l) >>> HPO42-(aq)
+ H3O+(aq), Ka2=
6,31×10-8, pKa2=
7,21
- HPO42-(aq) + H2O(l)
>>> PO43-(aq) +
H3O+(aq), Ka3=
3,98×10-13, pKa3=
12,67
For each of the dissociation reactions shown above, there is a
separate acid dissociation constant, called Ka1, Ka2, and Ka3 given
at 25 °C.
Aqueous solution.
For a given total acid concentration [A] =
[H3PO4] +
[H2PO4-] +
[HPO42-] +
[PO43-];
([A] is the total number of moles of pure H3PO4 which have
been used to prepare 1 liter of solution), the composition of an
aqueous solution of phosphoric acid can be calculated using the
equilibrium equations associated with the three reactions described
above together with the [H+]
[OH-] = 10-14
relation and the electrical neutrality equation. Possible
concentrations of polyphosphoric molecules and ions is neglected. The
system may be reduced to a fifth degree equation for
[H+] which can be solved numerically,
yielding:
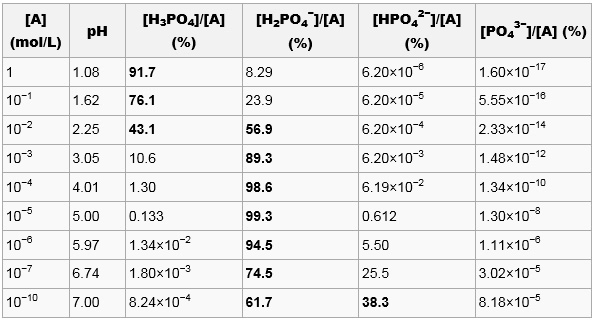
For strong acid concentrations, the solution is mainly composed of
H3PO4. For [A] = 10-2, the
pH is close to pKa1, giving an equimolar
mixture of H3PO4 and
H2PO4-.
For [A] below 10-3, the solution is mainly
composed of H2PO4- with
[HPO42-] becoming non negligible for
very dilute solutions. [PO43-] is
always negligible.
Since this analysis does not take into account ion activity
coefficients, the pH and molarity of a real phosphoric acid solution
may deviate substantially from the above values.
In water treatment, it is used by injection in the form of water
diluted reagent (solutions).
A formulation for the water
treatment.
Reaction mechanism of calcium bicarbonates (simplified
equations):
.....................
....................2H3PO4
+.......................3Ca[HCO3]2
. ......
>>>.... .........6
CO2 ..........+
6H2O....
+Ca3[PO4]2
(98x2=196)......
(162x3 or 30°F or 300 mg/L as
CaCO3)
..................(6x44=264)
.......
With 1 mg as H3PO4 added, there has
(30/98x2) = 0.15°F for lower TAC
(Alkalinity), and 1.347 mg as free CO2 and 0.97 mg/l as PO4
formed.
Storage.
A entreposer dans un récipient tenu fermé, portant une
identification claire de son contenu, placé dans un endroit
frais, sec et bien ventilé, à l'abri des bases, des
matières combustibles.
Uses.
(Phosphoric acid and its derivatives are pervasive and find many
niche applications)
- The dominant use of phosphoric acid is for fertilizers,
consuming approximately 90% of production.[
- wastewater treatment and industrial water
- Phosphoric acid may be used to remove rust by direct
application to rusted iron, steel tools, or other surfaces. The
phosphoric acid changes the reddish-brown iron(III) oxide, Fe2O3
(rust) to ferric phosphate, FePO4.
- In medicine:
- Phosphoric acid is used in dentistry and orthodontics as an
etching solution, to clean and roughen the surfaces of teeth
where dental appliances or fillings will be placed.
- Phosphoric acid is also an ingredient in over-the-counter
anti-nausea medications that also contain high levels of sugar
(glucose and fructose).
- This acid is also used in many teeth whiteners to eliminate
plaque that may be on the teeth before application.
Health-wise.
It can cause severe burns.
In soft drinks : Phosphoric acid, used in many soft drinks
(primarily cola), has been linked in
epidemiological studies to chronic kidney disease and lower bone
density.
Toxicological profile (French) - Acide phosphorique
(FT
37) par l'Institut national de recherche et de
sécurité (INRS).
Also, see NIOSH
Pocket guide to chemical hazards (The National
Institute for Occupational Safety and Health - US).
Sources : personal and Wikipedia,
the free encyclopedia.
 (use your browser)
(use your browser)

 (use your browser)
(use your browser)