Calcium carbonate
(limestone).
Chemical formula : CaCO3
(O = 47,96 %, C = 12 %, Ca = 40,04 %)
Molar mass = 100,087 ± 0,006 g·mol-1
Natural product :
It is a common substance found in rocks in all parts of the
world, and is the main component of shells of marine organisms,
snails, coal balls, pearls, and eggshells.
Calcium carbonate is the active ingredient in agricultural lime, and
is created when Ca ions in hard water react with carbonate ions
creating limescale (it is the hard, off-white, chalky deposit found
in kettles, hot-water boilers and the inside of inadequately
maintained hot-water central heating systems. In French :
tartre).
It is also often found as a similar deposit on the inner surface of
old pipes and other surfaces where "hard water" has evaporated. Other
than being unsightly and harder to clean, limescale seriously impairs
the operation or damages various components.
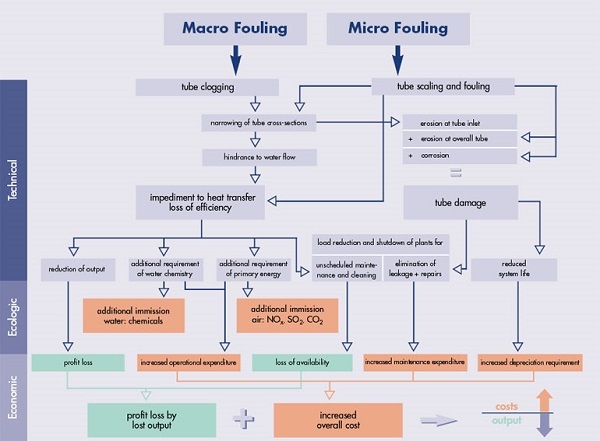
Fouling (corresponding effects of fouling) :
Precipitation fouling or scaling involves crystallization of solid
salts, oxides and hydroxides from solutions. These are most often
water solutions, but non-aqueous precipitation fouling is also known.
Precipitation fouling is a very common problem in boilers and heat
exchangers operating with hard water and often results in
limescale.
Piping, flow channels – reduces flow, increases pressure drop,
increases upstream pressure, increases energy expenditure, may cause
flow oscillations, slugging in two-phase flow, cavitation; may
increase flow velocity elsewhere, may induce vibrations, may cause
flow blockage

Polymorphs of calcium carbonate:
(polymorphism is the ability of a substance to crystallize in
different structures depending on ambient conditions).
Limestones represent 20% of sedimentary rocks.
Preparation.
The vast majority of calcium carbonate used in industry is
extracted by mining or quarrying. Pure calcium carbonate (e.g. for
food or pharmaceutical use), can be produced from a pure quarried
source (usually marble).
Alternatively, calcium carbonate is prepared from calcium oxide.
Water is added to give calcium hydroxide, and carbon dioxide is
passed through this solution to precipitate the desired calcium
carbonate, referred to in the industry as precipitated calcium
carbonate (PCC):
It is commercially available under different solid forms (depending on the manufacturer):
In water treatment; it is used by the percolation of water through a filter limestone (usually). Direct injection in powder form or in suspension has a low yield of neutralization.
NB - The concentration of calcium in drinking water (hardness) is
measured in French degrees (°F, 1 degree equivalent to 4 mg/L
Ca), also in mg/L as CaCO3.
The presence of limestone in the water shows no disadvantage to
health, on the contrary, and so there is no statutory maximum
content.
Properties :
Fine white powder; odorless, chalky taste
|
|
|
|
|
|
|
|
|
|
|
|
|
(in pure water) solubility product (Ksp, 25°C) |
4.8×10-9 |
|
|
|
To neutralize 1 mg of CO2, it is necessary to use (100/44)= 2.273
mg as CaCO3, which form (162/44)=3.68 mg calcium
bicarbonate [HCO3], and therefore also the neutralization of
1 mg /L to form CO2 (10/44)= 0.2273 °F/L asTAC (2.273 mg CaCO3/L
of Alkalinity)
Standardization.
Standard products used for the production of drinking
water: Official
French Bulletin - Calcium carbonate : NF EN 1018.
Uses.
Rocks made of limestone are used as follows:
Sources : personal and Wikipedia,
the free encyclopedia.
 (use your browser)
(use your browser)