|
|
To perform calculation of characterization, following five data are required:
Temperature, pH, conductivity (or
resistivity) must be measured "in situ" to avoid deterioration due to
transport of samples (risk degassing exchange with the ambient air).
This is true for freshwater especially and/or very aggressive
water.
Concentrations of chlorides and sulfates are out si you want to
calculate Larson-Index.
Note: concentrations of sulfate and chloride are given in
mg/L.
Temperature.
Temperature (°C) into the calculations of pK1, pK2 and pK, so in
those of pHs, IS and free CO2.
(see on this subject Equilibrium
file) and finally affects parameters of calcocarbonic balance.
pH.
pH represents the hydrogen potential ie the concentration of hydrogen
ions expressed by the negative logarithm of H+
(-H+)
Generally water is:
But it should be noted that pH of the calcocarbonic equilibrium
does not match pH of electronic neutrality (pH 7). It may have a pH
of equilibrium which is to be an acidic or basic pH of electronic
point of view.
Note: Hallopeau graph represents the "figurative point" of water in
question.
TAC.
TAC ("Titre Alcalimètrique Complet" in French) also called
Total Alkalinity or simply Alkalinity (Alk) is water content of
bicarbonates (hydrogencarbonates. HCO3-). carbonates
(CO32-), free alkali (OH-), and positive ions
associated with Ca2+, Mg2+, Na+ and
K+
Note : "Titre Alcalimétrique simple" as TA (not used herein)
measuring content of free water in alkali carbonates and alkali
(Na+ associated with sodium and potassium K+).
In natural waters TA can only occur si the pH is greater than or
equal to 8.3 (TA = 0 si pH <= 8.3).
The distribution of constituent ions for alkalinity can be calculated
from respective values of TA and TAC.
"Titre Temporaire" or dureté temporaire in
French : "Temporary title" or Temporary Hardness.
Also called carbonate hardness is often carbonates and
bicarbonates anions, mainly calcium and magnesium (anions disappear
after boiling water). We can sometimes find the term "alkaline
hardness."
Total Hardness
(dureté totale orTH = "Titre Hydrotimètrique" in
French).
corresponds to total amount of calcium and magnesium in water.
"Titre Permanent" ou dureté permanente : "Permanent
Title" or Permanent hardness (also called non-carbonated or
non-alkaline hardness) :
concentration of calcium and magnesium, other than carbonates or
bicarbonates. Thus mainly due to presence of anions sulfates.
chlorides and nitrates anions.
It is also dsiference between the total hardness and the temporary
hardness.
By definition, total hardness is equal to sum of permanent and
temporary hardness. Total hardness is also equal to sum of carbonate
and non-carbonate hardness.
Therefore, we have: permanent
hardness = TH - Alk. so Alk ± TH (which is general case of
natural waters almost).
By convention, these securities, which do not relate to a specsiic
ion, are always expressed in french degree.
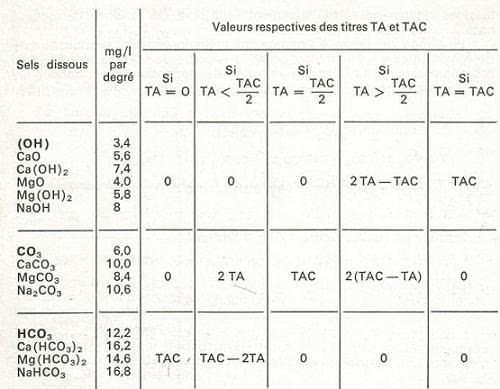
The following french table can deduct ions defining alkalinity of
water from TA and TAC values (general):

Calcium.
Calcium or calcic hardnesss is global concentration in calcium
salts. regardless of the associated anion. In french : Titre
Calcique [TCa] of water.
Also. Magnesium hardness (or Title Magnesia. TMG). only with
magnesium salts.
Total Hardness (TH) corresponds to total amount of calcium
and magnesium in water. Hard water is one leaves encrusting carbonate
deposits (scale generator) when heated. This feature is also
reflected on dsificulty of soap lather.
Waters are classified as follows generally:
Reminder to milliequivalents and French degrees (°F):
In analyzes, concentration of components is almost always expressed
in milligrams per liter (mg/L) or micrograms per liter
(mg/L)
for trace elements (1 mg/L=10-3
mg/L=10-6 g/L).
It is also sometimes, especially in US documents, from an expression
of the concentration in ppm (parts per million). Strictly speaking,
ppm refers to concentration of weight to weight. But there is not a
big mistake by equating as mg/L, or as g/m³ (in case of
extremely diluted solutions as natural water). Expression mg/L is not
always convenient to monitor results of an analysis. It should in
this case be replaced by milliequivalent per liter (meq/L).
By definition gram equivalent (g eq) is quotient of atomic weight for
simple substance considered. On the number of electric charge
(formerly the valence). For example, atomic weight of calcium is
about 40 and this body being divalent (Ca2+). The g
equivalent is therefore 40/2 or 20.
A solution with 1 g/L as calcium contains 1/20 = 0.05 equivalent/L,
or 50 meq/L. So the item is considered monovalent, e.g. sodium
Na+ mass 23, therefore the equivalent worth g as 23/1 = 23
g/L.
This notation has several advantages:
It allows summation of all elements analyzed. Which leads simply to
assess mineralization. ionic = balance and allows immediate
calculation of salt concentrations.
In water chemistry. we often need to know not details of various
ions. But rather the sum of some of them (Ca2+,
Mg2+, carbonates, bicarbonates, etc.).
It is for example titles: a measure expressed in mg/L would obviously
meaningless. While meq/L allows immediate evaluation.
However, an old habit was retained by French water caterers of
assessing these securities in French degrees
(°F).
We need to know : 1 equivalent = 5000 ° F. So, 1 meq/L = 5
°F (or 1°F=0.2 meq/L).
French degree is a concentration unit may be used as meq/L, to
express dose of any soluble salt in water. Widely used there few
decades, this notation is much less applicable than "securities" such
as TAC. TH. etc.
However, its use is still widespread in field of water treatment by ion exchange.
The units are as follows:
|
|
|
°dH or dGH |
°e or °Clark |
°US |
|
|
|
|
|
|
|
|
|
|
|
°dH or dGH |
|
|
|
|
|
|
|
°e or °Clark |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
as CaCO3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Al 3+ |
0.1111 |
8.993 |
BO2 - |
0.02335 |
42.82 |
|
Ba 2+ |
0.01456 |
68.68 |
Br - |
0.01251 |
79.92 |
|
Ca 2+ |
0.0499 |
20.04 |
Cl - |
0.0282 |
35.46 |
|
Cr 3+ |
0.05768 |
17.34 |
CO3 -- |
0.03333 |
30.01 |
|
Cu 2+ |
0.03148 |
31.77 |
CrO4 -- |
0.01724 |
58.01 |
|
Fe 2+ |
0.03581 |
27.93 |
F - |
0.05263 |
19 |
|
Fe 3+ |
0.0537 |
18.62 |
HCO3 - |
0.01639 |
61.02 |
|
H+ |
0.9921 |
1.008 |
HPO4 -- |
0.02084 |
47.99 |
|
K+ |
0.02558 |
39.1 |
H2PO4 - |
0.01031 |
96.99 |
|
Li+ |
0.1441 |
6.94 |
HS - |
0.03024 |
33.07 |
|
Mg 2+ |
0.08224 |
12.16 |
HSO3 - |
0.01233 |
81.07 |
|
Mn 2+ |
0.03641 |
27.47 |
HSO4 - |
0.0103 |
97.07 |
|
Mn 3+ |
0.07282 |
13.73 |
I - |
0.00788 |
126.9 |
|
Na+ |
0.04348 |
23 |
NO2 - |
0.02174 |
46.01 |
|
NH4 + |
0.05543 |
18.04 |
NO3 - |
0.01613 |
62.01 |
|
Pb 2+ |
0.009652 |
103.6 |
OH - |
0.0588 |
17.01 |
|
Sr 2+ |
0.02282 |
43.82 |
PO4 --- |
0.03159 |
31.66 |
|
Zn 2+ |
0.03059 |
32.69 |
S -- |
0.06237 |
16.03 |
|
|
|
|
siO3 -- |
0.02629 |
38.05 |
|
|
|
|
SO3 -- |
0.02498 |
40.03 |
|
|
|
|
SO4 -- |
0.02082 |
48.03 |
Dry Residue
(DR).
Dry Residue - measured after evaporation of filtered water and
steamed (drying) to 180 °C - assesses dissolved solids. It
allows to approach mineralization value. It can be deduced from
conductivity value (or its inverse, as resistivity):
Notes :
Conversion unit : resistivity (Ohm.cm) / conductivity (microsiemens /
cm)
 (use your
browser)
(use your
browser)