EquilWin
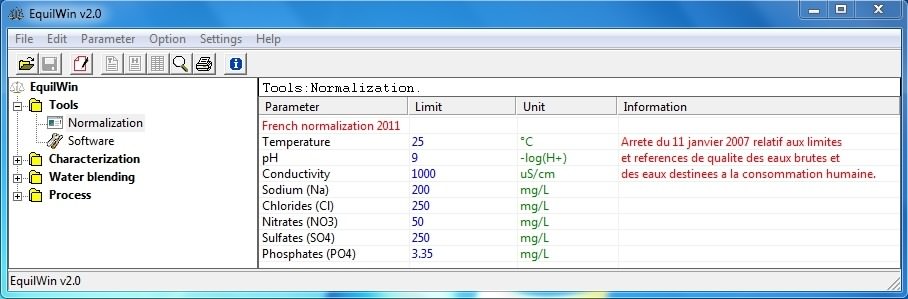
Allows to visualize (or not) French or international Standards, directly related to program.
Choice is made in Settings menu > Normalization...
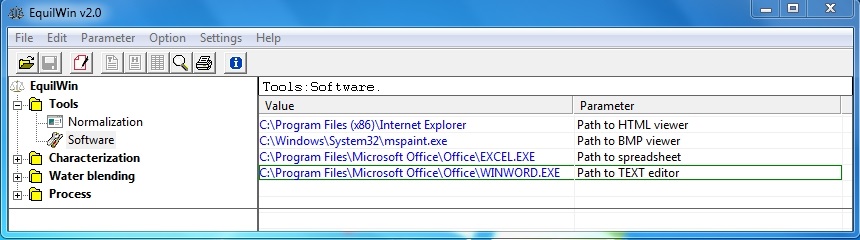
Allows setting the access path to executable programs used by software to specific actions:
-
display HTML pages,
-
viewing images,
-
use spreadsheets (Excel or other),
-
Using a text editor (such as Notepad/Winword or other).
Allows input data characteristics of water: reference and basic parameters required to calculations.
You can also set maximum saturation index that will be taken into account in calculations led to calcocarbonic water balance.
Also allows calculation of corrosion index (Larson index or Larson Ratio).
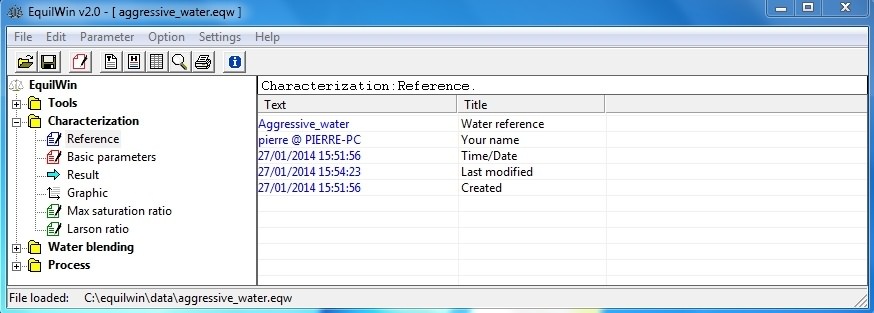
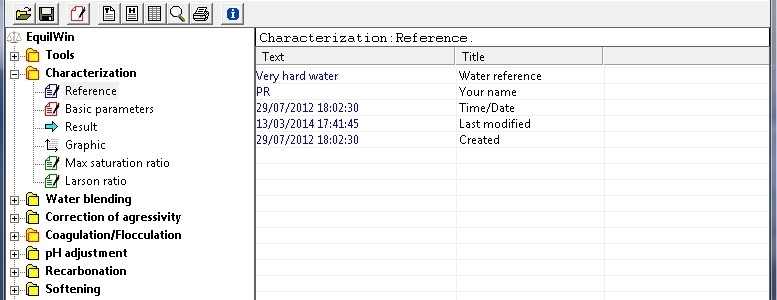
Writes reference to characterization of water.
Note: dates are saved automatically, but modified by editor. .
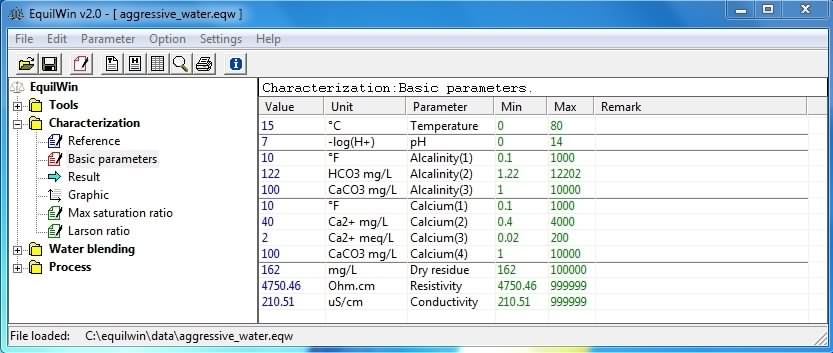
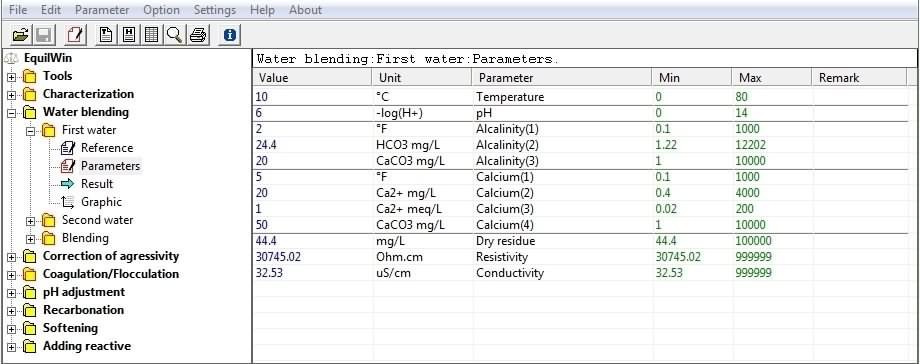
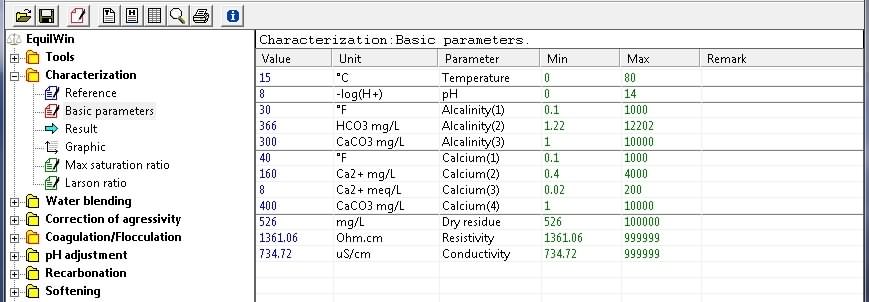
Allows entry of physico-chemical characteristics of under test water.
5 parameters are essential for calculations: temperature, pH, Alkalinity (French TAC), Calcium and DR (min-max limits have been set deliberately)
To do this, several ways of doing this:
Open a saved file > open File menu then Open characteristic ...
Open a previous prerecorded session > File > Open Session...
By 5 data entry into provided Value column (by clicking "?")
Furthermore,
Regarding TAC (alkalinity), 3 types of input possibilities available:
-
in French degrees (° F)
-
bicarbonate HCO
3
-
(mg / L)
-
calcium carbonate CaCO
3
(mg / L)
As regards calcium (calcium hardness), four possibilities of types of entries are provided:
-
French degrees (° F)
-
milligrams per liter calcium Ca
2+
(mg / L)
-
milliequivalents per liter of calcium Ca
2+
(meq / L)
-
calcium carbonate CaCO
3
(mg / L)
Regarding Dry Residue (DR), 3 types of input possibilities available:
-
milligrams per liter (mg / L)
-
resistivity (Ohm.cm)
-
Conductivity (mS / cm)
Note: For last three parameters, one of other input conditions (calculated automatically).
Also note that DR is calculated and displayed automatically (calculated from introductions of TAC and calcium).
|
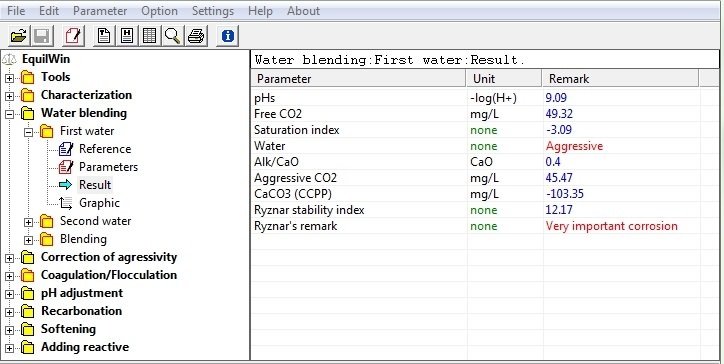
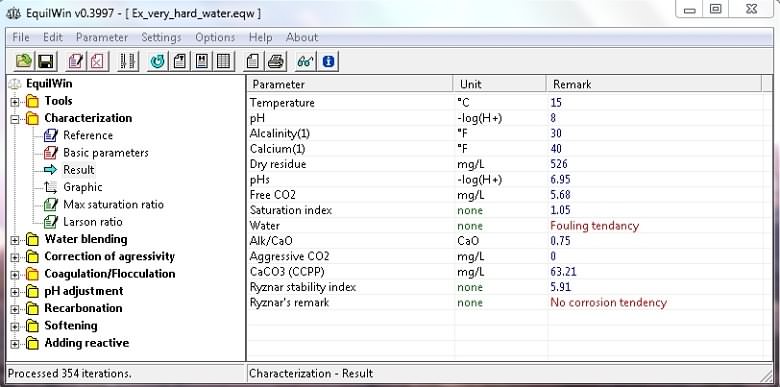
Results (characterization)
|
Allows visualization of calculations results of iterations equations based on Hallopeau / Dubin formulas (see appropriate documentation)
isan aggressive water for example:
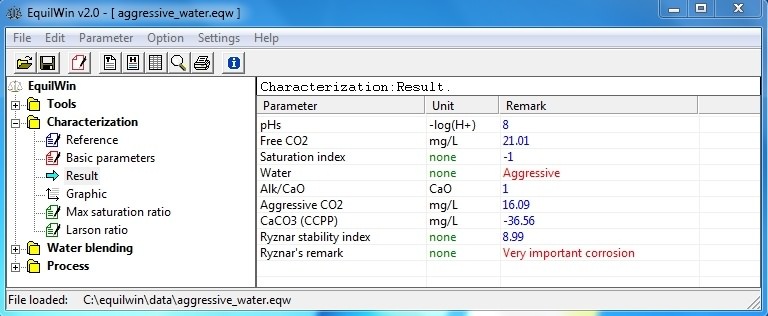
With,
pHs> saturation pH of calcium carbonate [CaCO
3
] or pH balanced water contact-solid calcium carbonate CO
2
free > mg / L of free carbon content (carbon dioxide) emissions.
Saturation index SI (or Langelier Index)> is water pH study - pHs calculated:
-
So SI < -0.1: aggressive water (opposite of limestone)
-
So SI > 0.1 : fouling tentancy
-
So SI => -0.1 and <=0.1 : calcocarbonic balanced
Nature of water > character of water (caused by previous calculation), indicating its behavior towards limestone: aggressive, scare-fouling or calcocarbonic balance.
Alk/CaO > ratio > alkalinity and calcium (as CaO). Give an indication of water composition:
-
If this ratio is smaller than 1 significantly, so is CaO is larger than Alk significantly, we can deduce that water contains a relatively large amount of calcium that is not in bicarbonate form : in general, it will be a water with high sulfate content.
-
If this ratio is greater than 1 significantly, so Alk is larger than CaO significantly, it means there bicarbonates other than calcium bicarbonate: in general, magnesium carbonate or sodium.
-
If this ratio is close to l, this means that we can consider all bicarbonate as calcium and there is little or no other calcium salt (water course may contain salts other natures: magnesium sulfate, sodium chloride, etc.). This is the most common freshwater case.
Aggressive CO2> mg / L content of carbon aggressive towards limestone [CaCO
3
] dioxide. It represents difference between free water and CO
2
free C02 equilibrium water to CaCO3 (CO
2
called "balancing"). This is fraction of free C0
2
is likely to attack limestone, also, combine lime to dissolve in form of bicarbonate.
Note: if the water is to balance or scale-forming, one = 0.
CaCO3 (CCPP) > limestone amount (CaCO3 as mg / L) be dissolved potentially by aggressive CO
2
. This value is equal to 0 for water in equilibrium and for fouling water (or encrusting) indicated precipitated CaCO
3
.
Stability index (according Ryznar) > is 2pHs - pH.
Indication of trend to Ryznar > gives an indication of corrosive or encrusting trend (scale-forming) water.
( see links on
documentation
for information more )
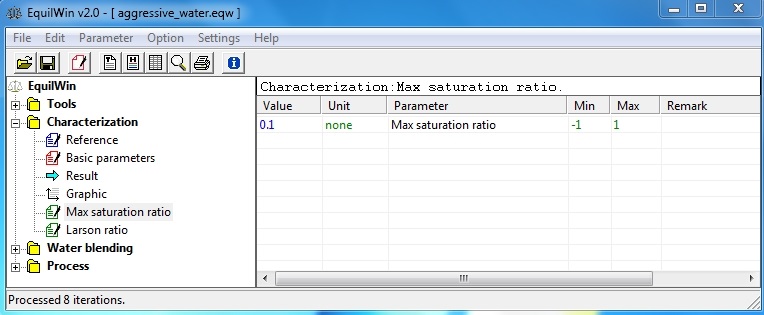
This entry worth a maximum saturation index means that during an equilibration requested, this index will be considered stopping calculation "equilibrium" and it can ask to obtained after correction processing of aggression that judgment is with an index < 0.1 (ie, aggressive water) or > 0.1 (ie, scale-forming water).
By default it is 0.1, and minimum and maximum limits are -1 to +1.
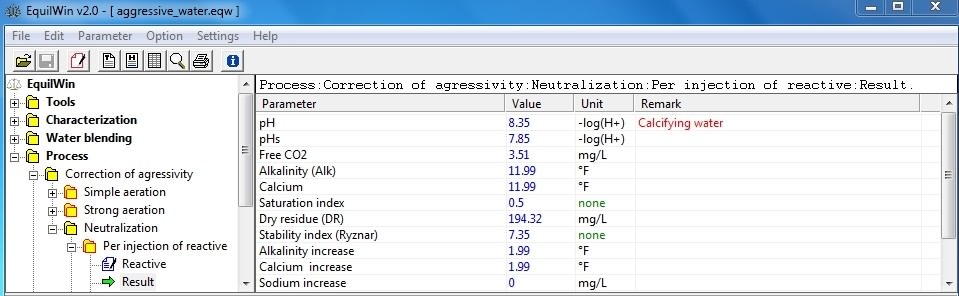
Example (max SI = 0.5, and neutralization with lime to an aggressive water) :
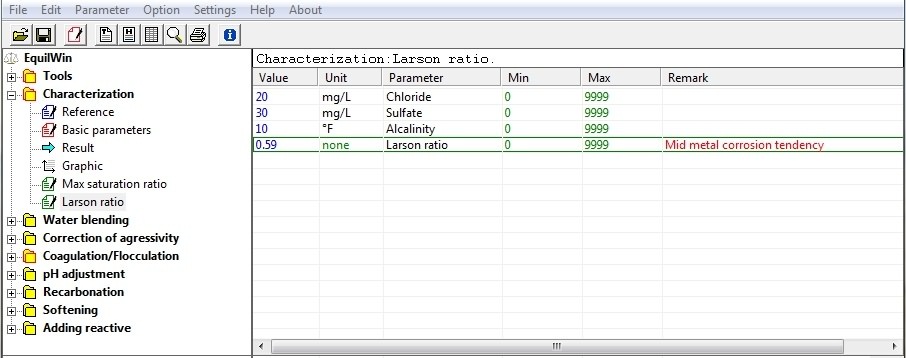
Index Larson (Larson Ratio) is an indicator of corrosion index with respect to metals.
It is defined as [(2 x sulfates] chlorides +] / alkalinity (TAC). Values as mole / liter.
Example:
( see links on
documentation
for information more )
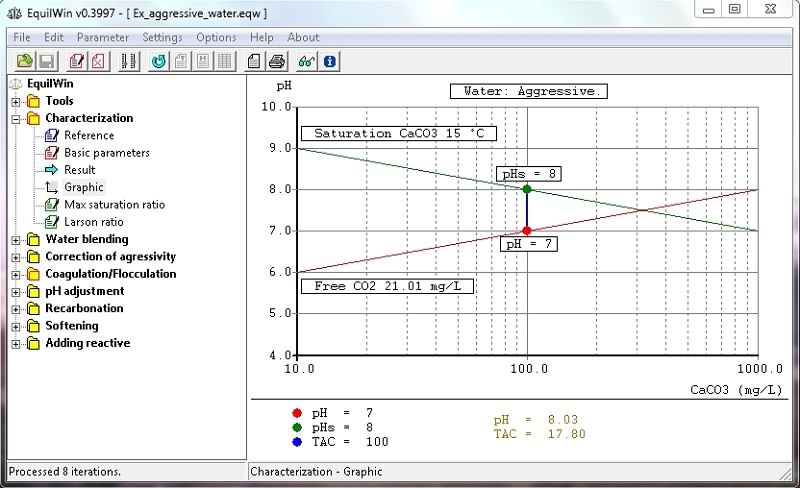
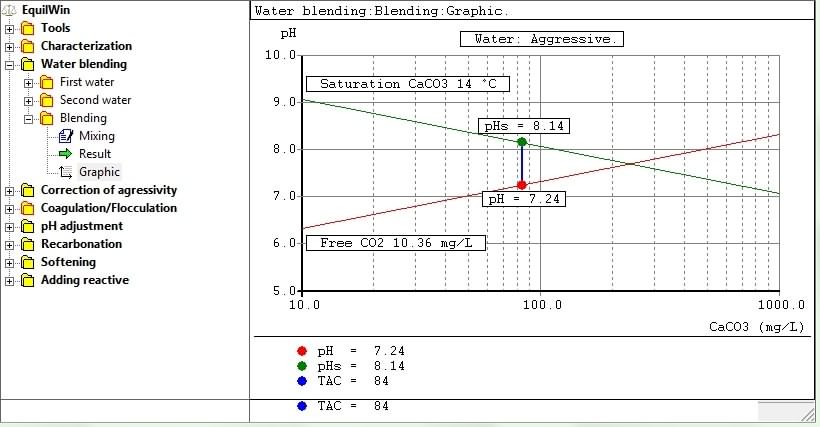
Hallopeau Dubin-graph visualization according to characterization of water under test or result of processing.
Graph constructed with logarithms of alkalinity and pH as abscissa ordinate.
Including:
-
saturation right of calcium carbonate [CaCO
3
] > depending on temperature (shown), the dry residue and Alk / CaO considered value,
-
free CO
2
right (or carbonic acid H
2
CO
3
) > function alkalinity (TAC), pH and pK'1, so DR and temperature (free CO
2
indicated value)
-
pH > pH of water under test (or "representative point" of the water in question),
-
pHs > calculated saturation pH (pH equilibrium solubility of CaCO
3
water considered)
Reminder:
Editor can parameterize it: change of scale to min / max values for abscissa and ordinate of TAC unity (°F default) > position on graph and right mouse button, or menu parameters > Graphics ...).
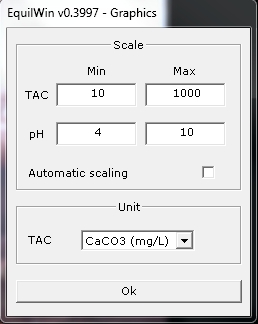
- You can also edit graph. Example of previous aggressive water):
This tool allows to characterize a water obtained by mixing two water (but not limited in number) indicating respective percentages of each in mixing water. Waters can be contained in features or recorded sessions already, or with additional water.
In practice, if you want to experiment mixing from same waters and keep the calculations proceed as follows:
Make a first mixture by ensuring that all water used are recorded and accessible therefore by "File / Open characteristic..." menu. Then save the calculation of mixture, or record session> "File / Save session..."
Make all other tests mixing launching every time a new mix and reminding waters with "File / Open characteristic..." menu. Each calculation can be saved under as different name.
Note that calculation is performed only if sum of percentages of two waters is 100%. Furthermore, a graph is used to display water mixture obtained.
Calculation results of mixture can be sent in
Characterization
of water using the menu "Copy to basic parameters" and it is possible to apply treatment to this water by launching processing tools.
Writes reference to characterization of first water (so it is a new water).
Note: dates are saved automatically , but modified by editor.
Allows parameters introduction of "
First Water
".
When you click on button "First water," 3 possibilities:
-
water to be added is that characterization of under test water, and only results window it using drop down menu and select "Copy to first water" menu (with right mouse button)
-
retrieve characteristics of saved water, then it is selected in window (right-click) menu with "Read from file"
-
either you enter data as for characterization. In this case, save water with "Copy to basic parameters:." menu or "File / Save session..." when screen is on "Results."
Example :
Allows to visualize calculations results of First Water. Note: same parameters given to results of water characterization.
(following entries as previous example):
Writes reference to water characterization of during Second (so it is a new water)
Note: dates are saved automatically, but modified by editor.
|
Parameters (second water)
|
Allows introduction of parameters to"second water".
When you click on "second water" button , 3 possibilities:
-
or water to be added is that characterization of under test water, andresults window only it using the drop down menu and select "Copy to second water" menu (right mouse button)
-
or retrieve characteristics of saved water, then it is selected in window (right-click) menu with "Read from file"
-
either you enter data as for characterization. In this case, it is prudent late entries, save water with "Save characteristic..." menu or "File / Save session..." when screen is on "Results."
Example:
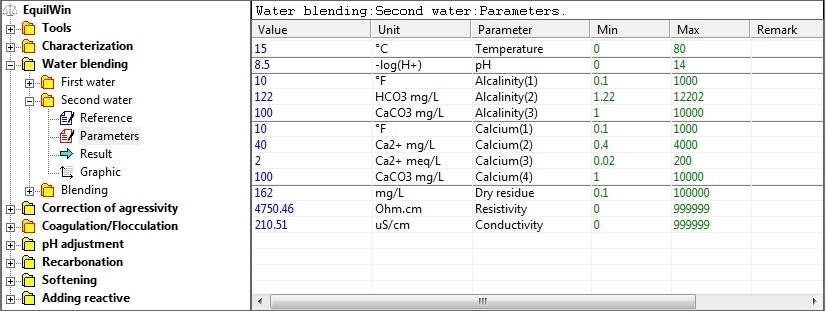
Allows to visualize results of calculations of second water.
Note: window of identical parameters to characterization results.
(following entries in previous example):
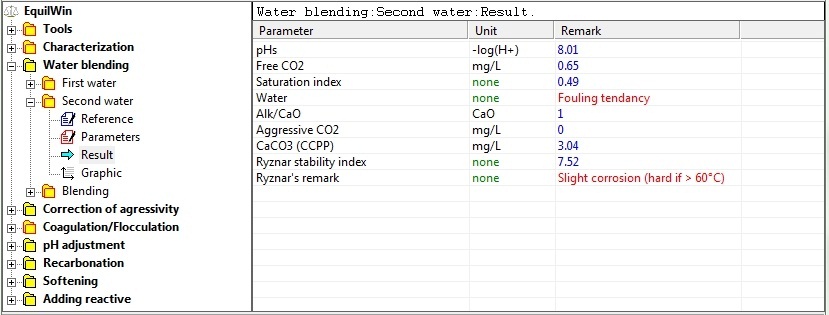
Enters fraction % of mixture (only one of two water which causes % calculation of other water ).
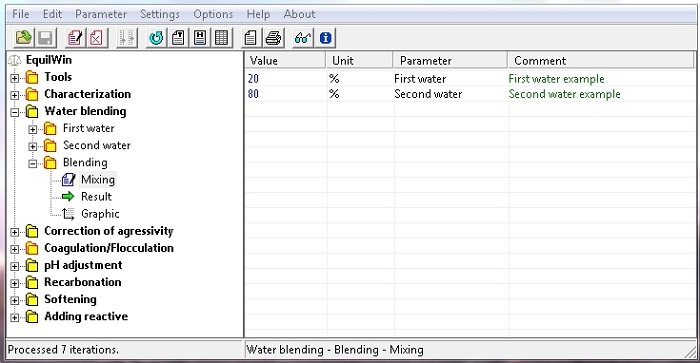
Allows results visualization calculations of two waters mixture.
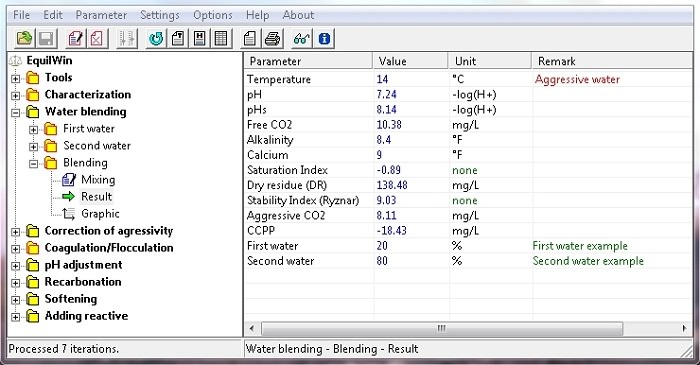
Allows graph visualization of two waters mixture.
Most proposed treatments are those that are usually found on plants for drinking and industrial water production. Obviously these methods are used in software to show their impact on balance <> CO2 <> Bicarbonates <> Carbonates <> Calcium, and the consequence thereof on behavior and nature of treated water.
|
Correction of aggressivity
|
Can change the nature of water if it is aggressive, ie succeptible etching on limestone and causing technical problems for collection, conveyance and distribution of water, and even some books (see this
particular link
).
The aim is to eliminate carbon dioxide (CO2) aggressive water, and optionally if necessary to increase alkalinity (TAC) and / or calcium hardness (TH) thereof.
To do this two types of corrective treatments are offered: aeration and neutralization (reactive neutralizing action).
Note that remineralization of water (given other treatments) is also possible.
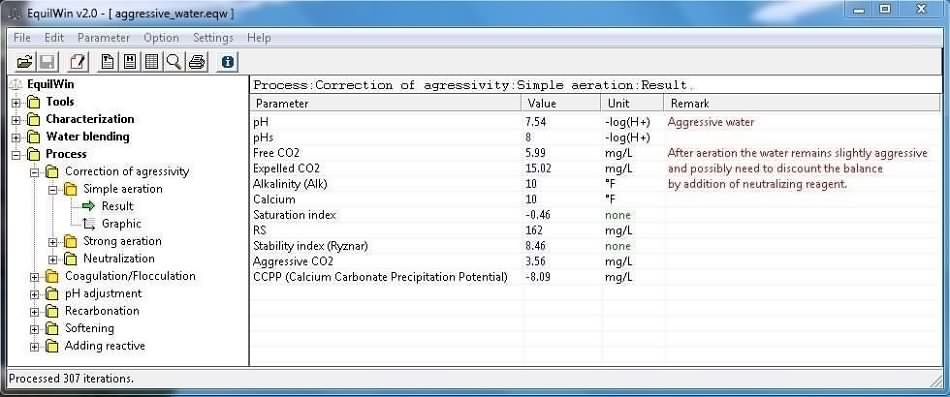
Eliminates dissolved aggressive carbon dioxide by CO2 escape .
This process does not reduce CO2 short of 6 mg / L (ie stop calculations to this value).
(see link
Documentation
on line for more information).
Example of results corresponding to under test water (Characterisation).
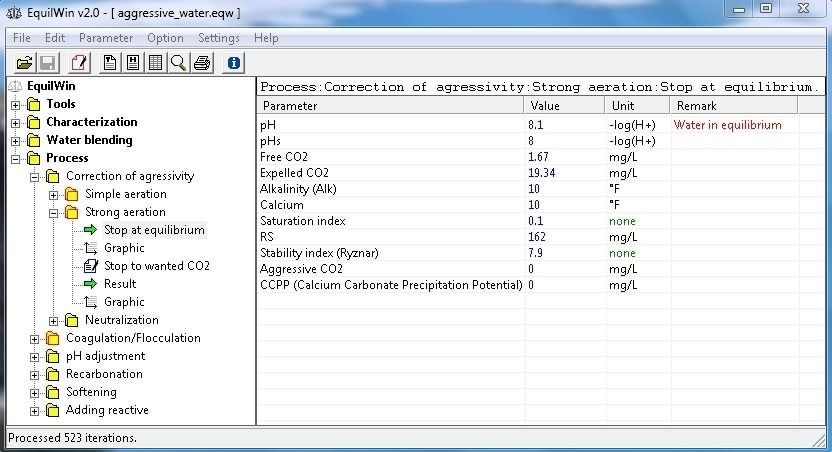
Two options to choose from for this treatment:
-
Stop at equilibrium > means that calculation is performed until calcocarbonic water balance,
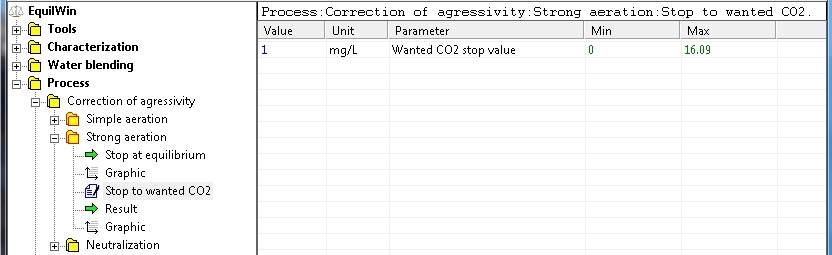
-
Stop to wanred CO2 > means that editor to choose free CO2 content to which he wants to stop calculations.
Example of results corresponding to under test water (Characterisation).
Choosing wanted CO2 (value): for example, 1 mg / L (upper limit value is indicated):
Example of results corresponding to under test water (Characterisation).
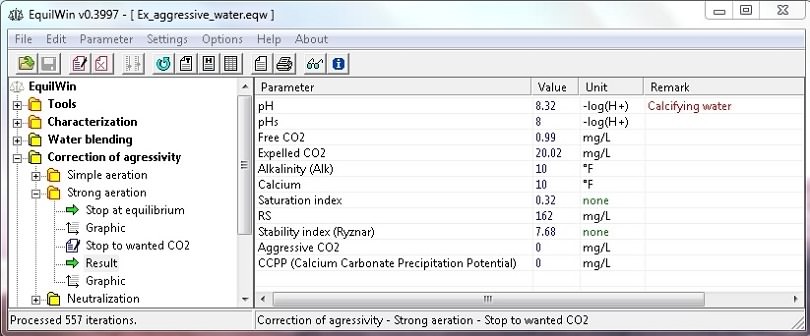
Helps neutralize aggressive action with alkalizing reagents.
So as to do:
-
per injection of reactive (four products, including solid or liquid)
-
per filtration (percolation) on a base material limestone (CaCO3)
Introduction of
purity
of pure reagent (0 to 100%) solids to 4, and in grams per liter (g / L) for liquid solution (lye, up to 762.5 g / L).
In this example, choice of slaked lime, with a purity of 85% (to 100% if unknown):
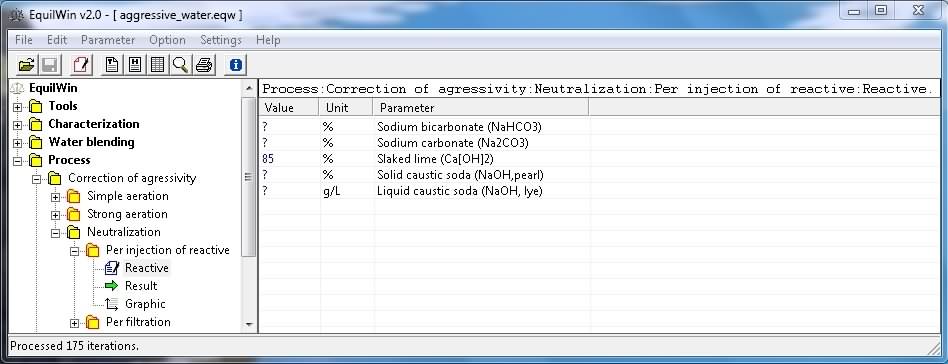
Display results corresponding to under test water (Characterization example) per action of slaked lime with 85% purity:
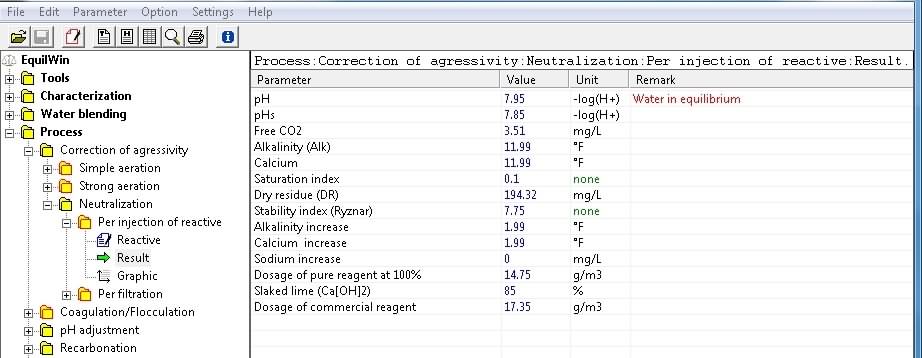
Note: it should be noted that rate of treatment of commercial reagent, ie technical lime 85% cleanliness Ca [OH] 2, is calculated and displayed.
In this example, choice of CaCO3 filtration , with a purity of 90% (= 100% if unknown):
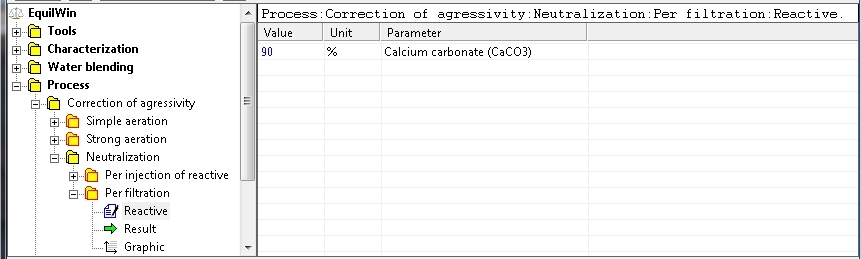
Display results corresponding to under test water (action of CaCO3 and purity 90%):
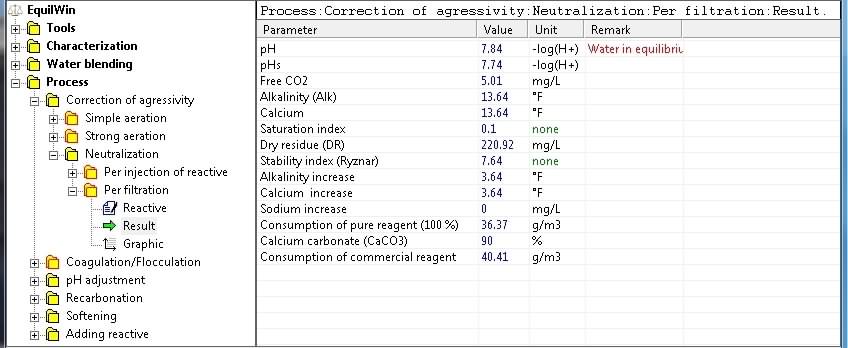
Note: we note that
rate of consumption
of commercial reagent (media), ie calcium carbonate technical purity CaCO3 90%, is calculated and displayed.
Allows you to verify change of water nature if it is treated with a reagent coagulation.
The action of these reagents always bring destruction bicarbonate / carbonate (ie a decrease in alkalinity) and correspondingly carbon dioxide increasing : water can become aggressive (if not already!).
(see this
particular link
)
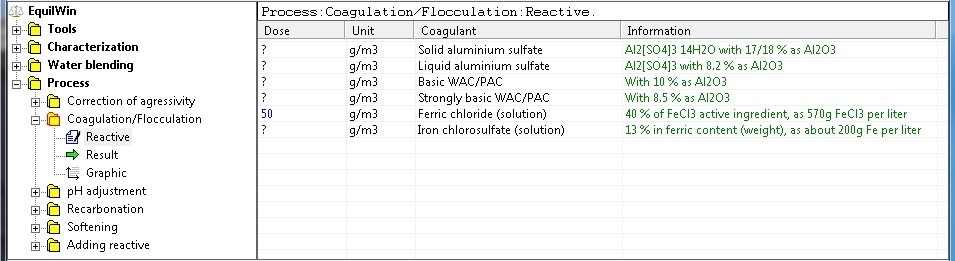
To do this six types of coagulants are available:
|
Reactives (Clarifying agents)
|
In this example, selection of ferric chloride solution, with a treatment dose of 50 g per m3 of water:
Display results corresponding to under test water (Characterization example),
(and action of ferric chloride with a treatment dose of 50 g/m3):
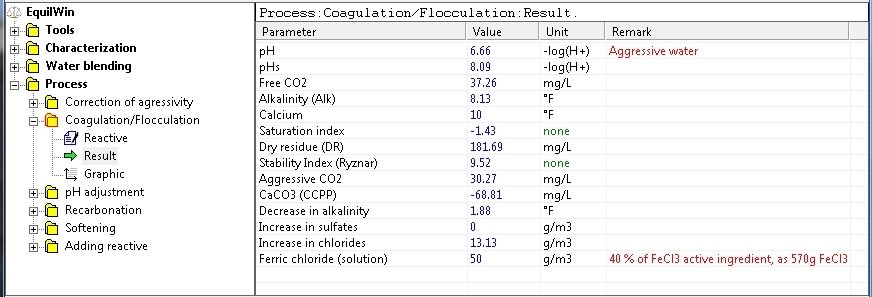
Note decrease in TAC (alkalinity), increased aggressive CO2 (+ 9.26 mg / L) and chloride (+13.13 mg / L).
This treatment allows to check the nature of the modification of water if pH adjustment of water is requested by adding acid.
(If the pH is less than the desired pH of the water in question) or addition of a basic reagent (if the pH is higher than the desired pH of the water in question).
Note: depending on the selected pH, the software offers an
acidification
or
alkalinization
.
(see the links on the
Documentation
for more information).
In this example, selection of pH adjusted to pH 6 (wanted pH):
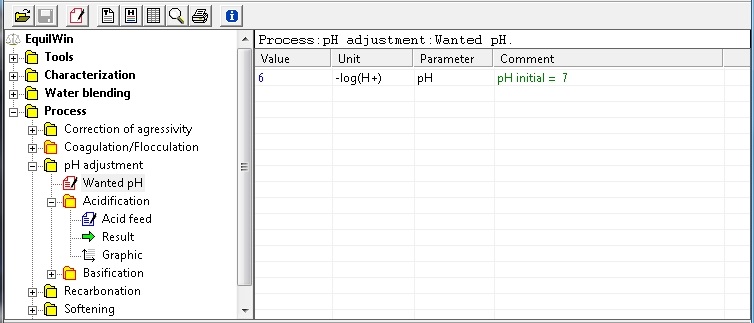
|
Acidification (Acid feed)
|
Choosing proposed introduction of acid and purity of pure product (in %).
Note: initial pH of under test water and desired memory are recalled (Comment column).
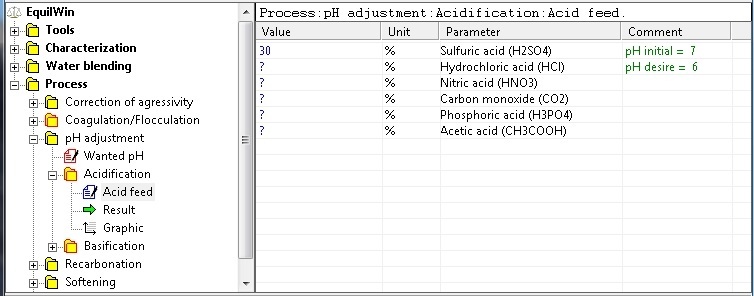
|
Result
(Acid feed to wanted pH)
|
Display results corresponding to test water (Characterization), and action of chosen acid):
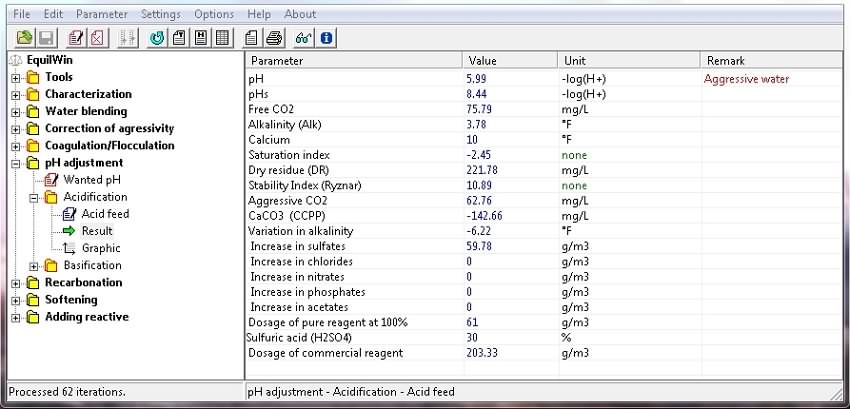
Note decrease in TAC (alkalinity), increased aggressive CO2 and sulfates.
Furthermore, the acid rates to obtain wanted pH are shown, recalling contents of pure product (purity) and rate technical product (commercial reagent).
In this example, selection of pH adjusted to pH = 7.9 (wanted pH):
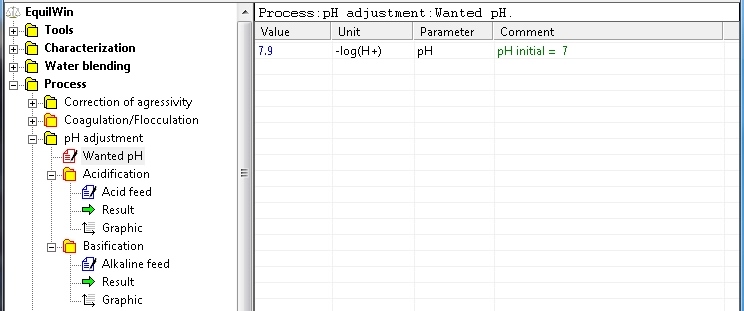
|
Basification (Alkaline feed)
|
Choice of alkalines and proposed introduction of purity pure product (in %).
Note: initial pH of under test water and desired memory are recalled (Comment column).
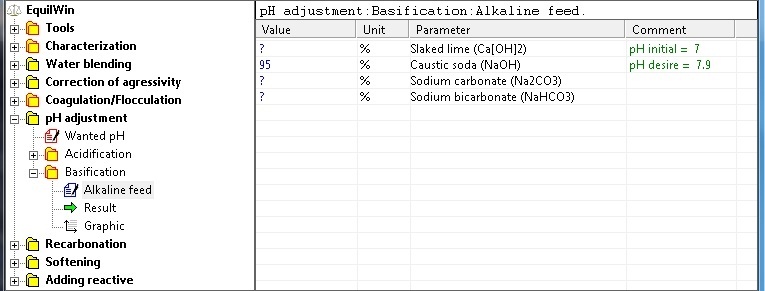
|
Result
(Alkaline feed to wanted pH)
|
Display results corresponding to water under visible in test characterization (and action of selected alkaline):
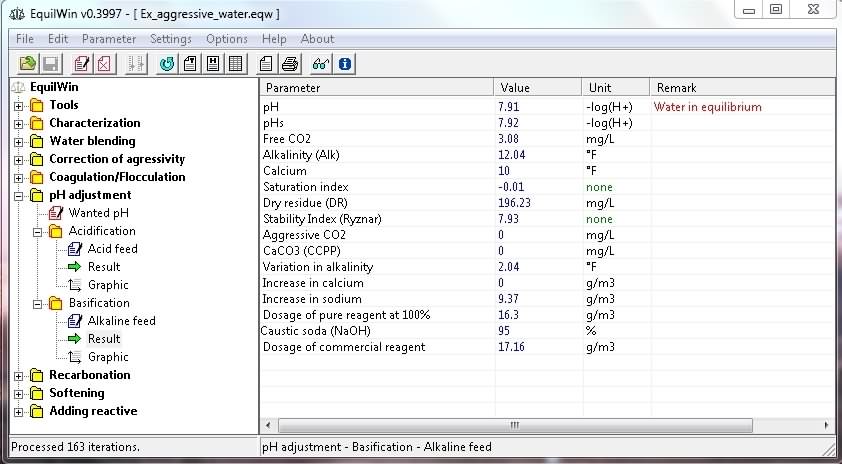
|
Recarbonation
(remineralization)
|
This treatment can impart desired characteristics to optimum fresh water containing little or no carbon dioxide, and moreover that supersaturated water at pH greater than 8.3. To achieve desired result, it is necessary to apply to these waters a remineralization treatment that will give them a mineralization higher than would be obtained by an elementary neutralization treatment, carbonated or not. The aim is to increase the content of calcium bicarbonate (alkalinity and calcium increase ) reacting an alkaline reagent (lime) on carbonic gas previously introduced.
(see
Documentation
link for more information)
Two great options (and sub-options) are available:
-
partial recarbonation : injection of CO2 carbon dioxide and Ca [OH] 2 lime, stopping has desired TAC (Alkalinity),
but also at desired pH (below to equilibrium pH achieved by a total remineralization).
-
total recarbonation (full-scale):
-
Injection of CO2 carbon dioxide and Ca [OH] 2 slaked lime,
-
Injection of CO2 carbon dioxide and fitration on a base material limestone (CaCO3).
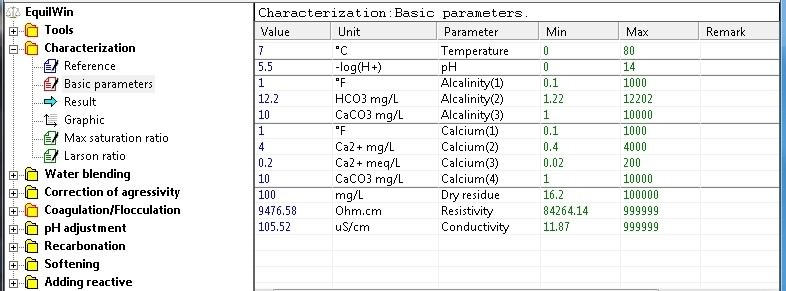
An example is given from a very soft water and aggressive (Alkalinity = Calcium = 1 ° F)
reference:
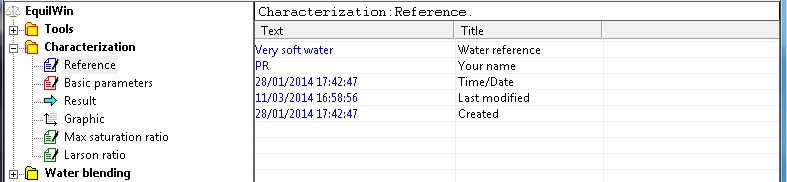
Basic parameters:
Results:
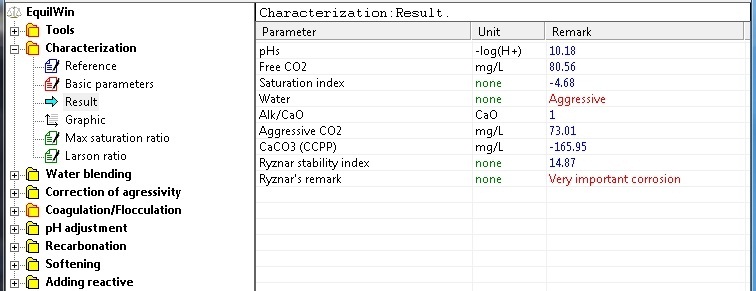
|
Partial recarbonation
(remineralization)
|
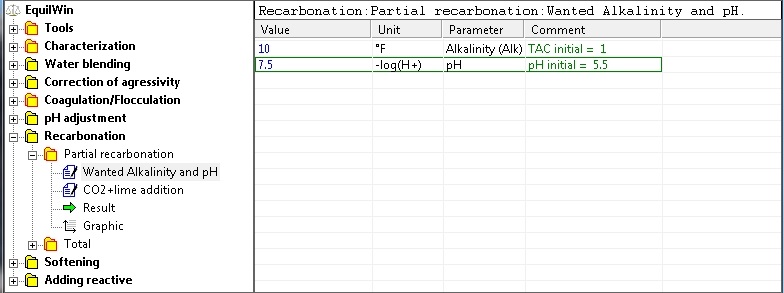
It is therefore partially remineralize water thus stopping the injection of neutralizing reagent (in this case slaked lime) at a given pH (desired pH).
Introduction of necessary parameters, or desired TAC (> considered water alkalinity) and stop has desired pH (> pH of water in question).
For our example of very soft water we chose: TAC = 10 ° F and pH = 7.5
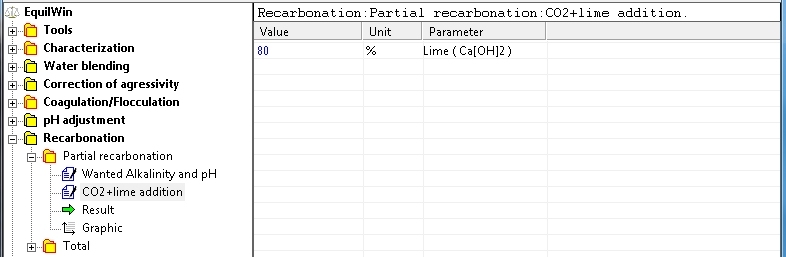
Introduction purity of pure reagent (0 to 100%) for lime. In this example, purity = 80% (if unknown to 100%):
|
Result (partial recarbonation)
|
Display results corresponding to the water under visible in our test sample characterization.
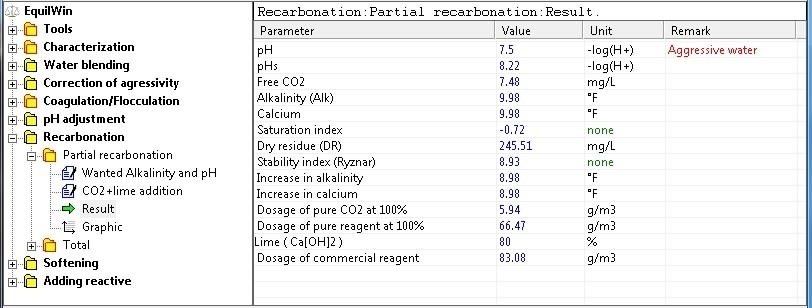
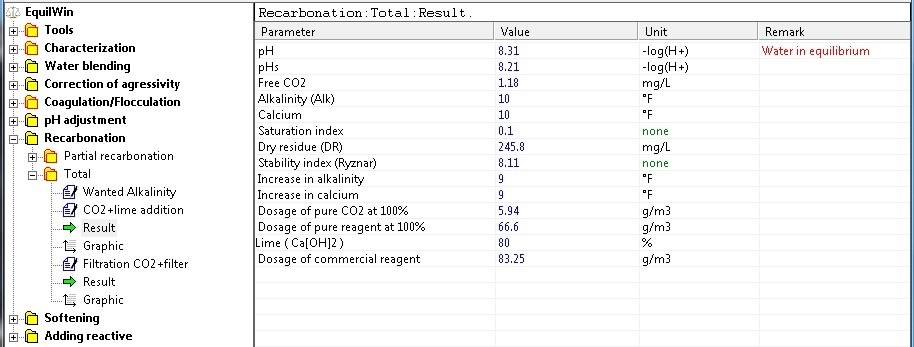
Note: treatment rates to implement (in pure and commercial reagent) indicated. And nature of water (balanced or aggressive).
|
Total recarbonation
(total remineralization)
|
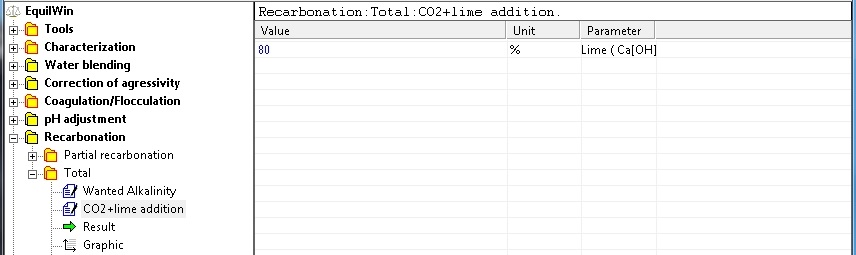
Choosing a total remineralization per CO2 carbon dioxide + Ca [OH] 2 lime addition.
In this option the pH is not introduced, calculations stop when water is balanced.
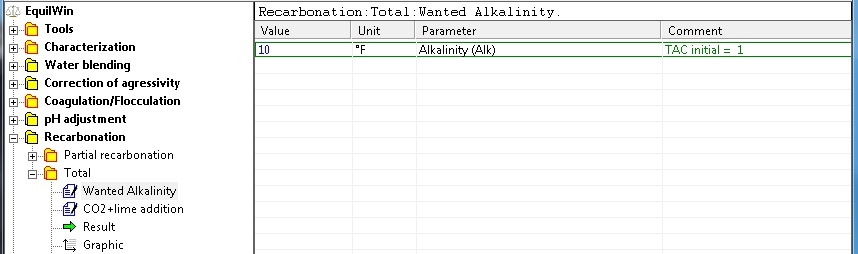
Introduction of the desired alkalinity (TAC in °F). For our example: TAC = 10 ° F (> the initial TAC water under test is reminded) .
Introduction of purity of pure reagent (0 to 100%) for lime. In this example, purity = 80% (100% if unknown):
|
Result
(total remineralization)
|
Display results corresponding to the water under visible in our test sample characterization.
|
Total recarbonation
(total remineralisation)
per filtration
|
Choosing a total remineralization by injecting CO2 carbon dioxide and fitration with a base material limestone (CaCO3).
>3 filtration options are available:
-
percolation through limestone
-
percolation through non-calcined dolomite
-
percolation through calcined dolomite
For our example, choice of limestone filtration (calcium carbonate) with 90% purity as CaCO3.
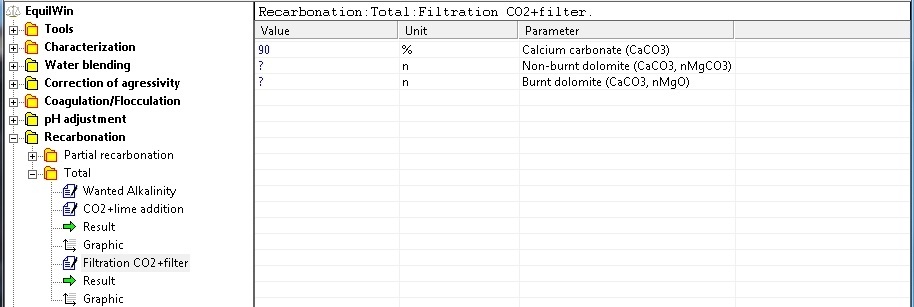
NOTE: If you selected Dolomites, it is necessary to introduce n factor (from 0.1 to 1 maximum), corresponding to the fraction of carbonate magésium (MgCO3 ) or dioxyde magnesium (MgO) present in the dolomite.
|
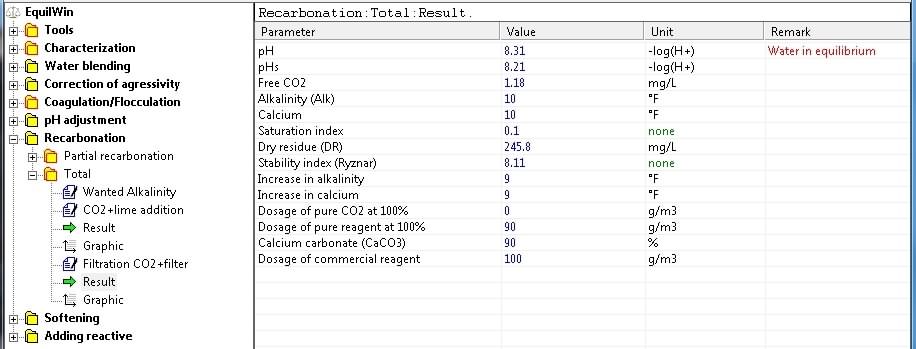
Result
(total remineralisation)
|
Display results corresponding to the water under visible in our test sample characterization.
Reduce Total Hardness (or Total Permanent Hardness) of hard water per softening treatment, ie in general, from calcium hardness superior to 300 mg/L as CaCO3 (30 °F) - (see
Documentation
link for more information.)
To do this, two main options are available:
-
softening by
injecting an alkaline reagent
, and decreased Calcium Hardness (calcium) by precipitation of calcium carbonate: 3 reagents basic (alkaline): lime, solid sodium hydroxide (caustic soda) and sodium hydroxide solutions,
-
softening by percolation on ion exchange resin (cationic resins), and decrease the Calcium Hardness (calcium) or Total Hardness (calcium + magnesium) by fixing ions (cations) on the types of resins.
An example is given from a very hard water (Alkalinity = 300 mg/L as CaCo3 (TAC = 30 °F) and Calcium = 400 mg/L as CaCO3 (40 ° F). or,
Basic parameters :
Calculation results:
|
Softening per reagents injection
|
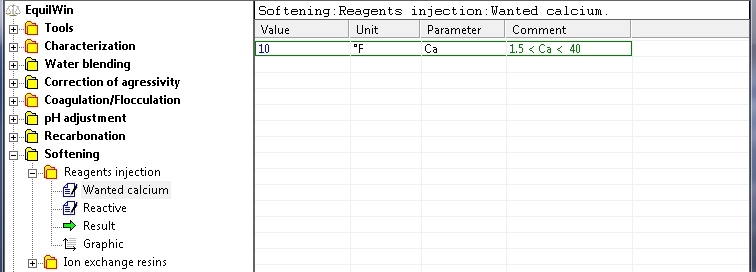
Choice : softening per reactive injection.
Introduction of the wanted calcium (limits indicated in comment), for example: 10 °F as Ca [Calcium or Calcium Hardness) or 100 mg/L as CaCO3:
Choice of alkaline reagents: slaked lime or soda (in pailletttes) or liquid (solution) .
Introduction of the purity of the solid product: as% of Ca [OH] 2 for lime, in% NaOH for sodium hydroxide flake (0.1 to 100%), or in grams of NaOH per liter (g / L) for sodium hydroxide solution (in the latter case the limit is 762.5 g / L).
For example, choice of solid soda (flakes) with a purity of 95% as NaOH: 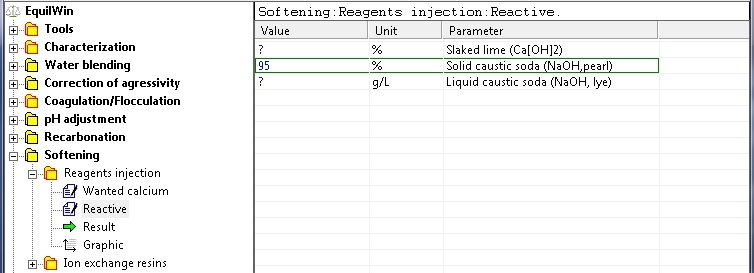
|
Result
(softening per injection)
|
Visualization of calculation results (as example and choice):
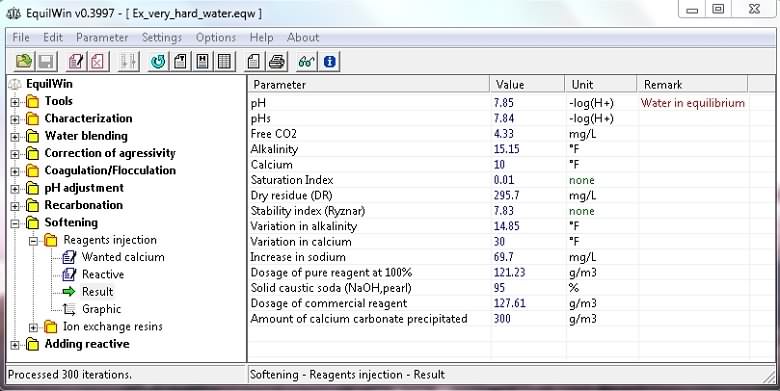
Note: The treatment rate of used reagentsare (pure and commercial) is indicated, and the amount of precipitated calcium carbonate as gCaCO3/m3 for treated water (the weight of calcium therefore mud).
|
Softening per ion-exchange resin
|
For this option further data are needed for calculations:
-
one hand, the respective values of [Total] Hardness (TH, in °F) to under test water, and desired Hardness after treatment,
-
secondly, to cation exchange resin, the
Total cation-exchange Capacity
, expressed as equivalents per liter of resin (eq/L R).
(see links on
Documentation
for more information).
For rest of example: introduction of total hardness (known and desired). The low and high limits are mentioned).
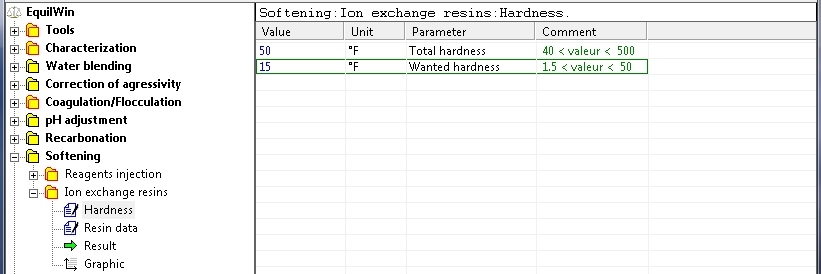
Introduction of the total fixing capacity of resin (Data): 1.8 eq / LR for example:
Note: input 1.5 if unknown (lower limit)
.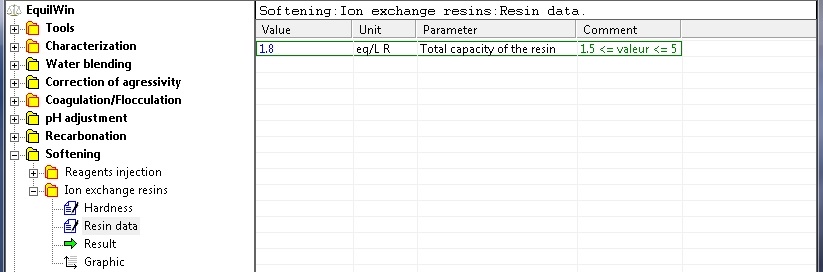
Note: limit value specified for a conventional total binding capacity of cation-exchange resin (sodium form).
|
Results
(softening per ion-exchange resin)
|
Display results for the given example:
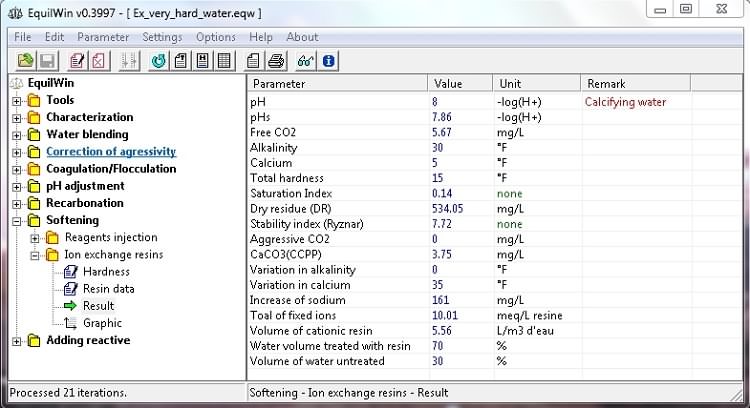
Note that the calculated volume of resin required for binding of (potential) Ca+Mg ions is given in liters of water per m3 of resin to be treated.
Furthermore, for reasons of economy it is calculated % of water, actually percolate on resin and the drifting or "by-pass" (ie untreated resin).
Used to test the water in question (shown in Characterization) of standard additions (by injection) of certain reagents, and check the action of these reactives on water balance.
or,
-
adding calcium (two calcium reagents are available)
-
adding an acid (six acid reagents)
-
adding a base (four alkaline reagents)
-
adding chlorine (chlorine gas and / or concentrated or diluted chlorine solutions)
Note: it can not be done if adding calculations may soften the water: the case of water at equilibrium or entartrantes which we would like to add a base).
To serve as an example, we return to that given for Characterization (aggressive water).
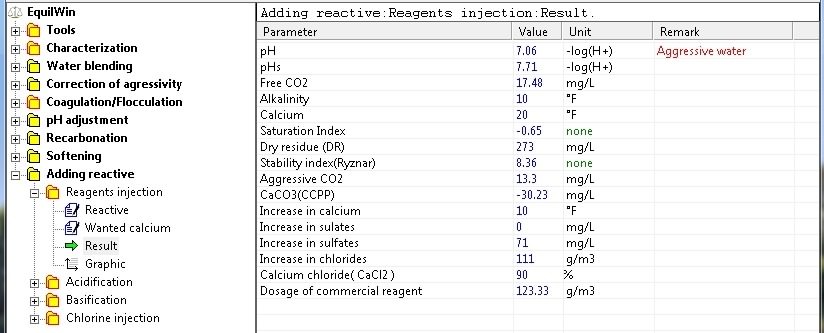
Increases calcium of water (calcic hardness) without increasing alkalinity (TAC).
|
Choice of
calcifying reagent
|
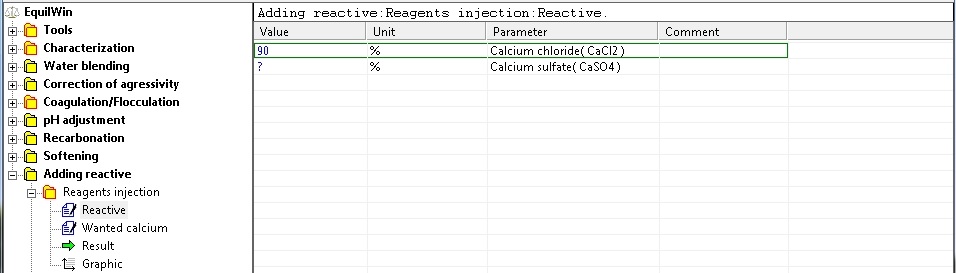
Calcifying reagent: calcium chloride or calcium sulfate (see
Documentation
link for information more).
Introduction of the purity of solid: in % as CaCl2 for calcium chloride and % as CaSO4 to calcium sulfate (% 0.1 to 100).
For our example, choice of calcium chloride with a purity of 90% as CaCl2:
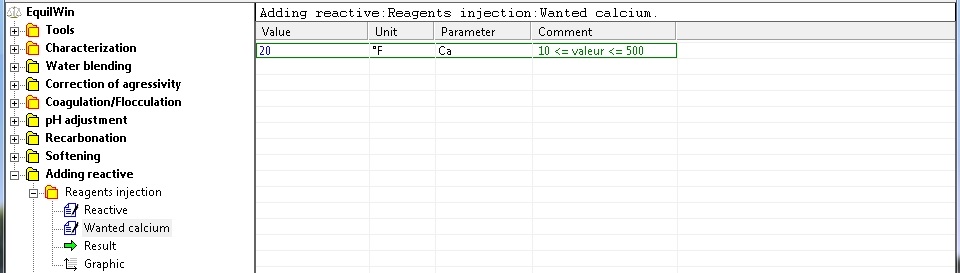
Selection of the wanted calcium as °F (or calcic hardness, calcium or TH)
(for our example, entering 20 °F value).
Note: be limits are shown, 10 °F (lower limit corresponding to the calcium of treated water) up to 500 °F
(we consider the upper limit is not exceeded, however we recommend a limit of 50 °F).
|
Result (calcium injection
|
Display results for example of the water to be treated:
Used to test the variation of parameters of water by action of adding acids, so in particular the decrease in pH and alkalinity (TAC) and increased aggressive CO2 (among others).
|
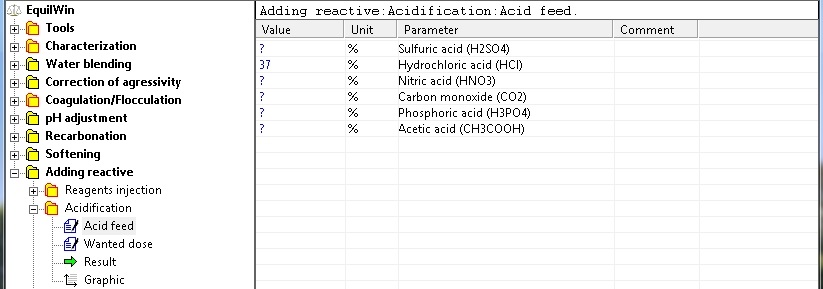
Choice of acids (acid feed)
|
Choice of acids and introduction of pure product purity (as %):
> For our example: hydrochloric acid at purity 37% as HCl (technical acid density 1.19).
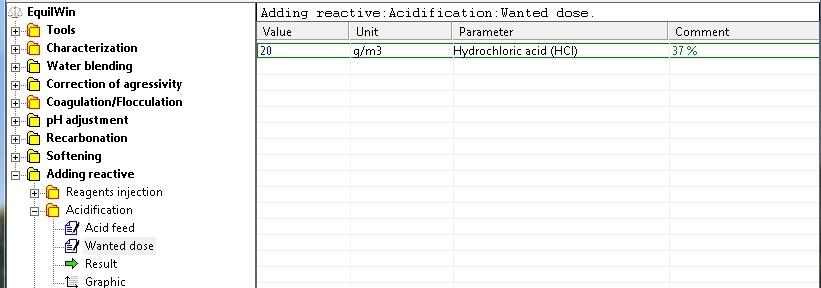
Entrance acid wanted dose: an example with 20 g per m3 of water (recall the purity Comment).
Display results for example of water to be treated:
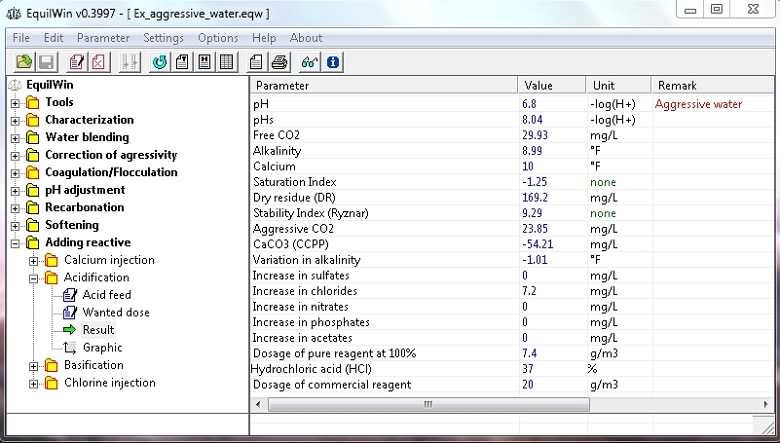
Note: increased aggressive CO2 (+ 7.76 mg / L) and CCPP (+ 18.65 mg / L) and chloride ions for choice of HCl (hydrochloric acid).
|
Basification
(Alkaline injection)
|
Used to test parameters variation of water by action of adding alkaline reagents, so in particular increase in pH and alkalinity (TAC) and lower aggressive CO2 (among others).
|
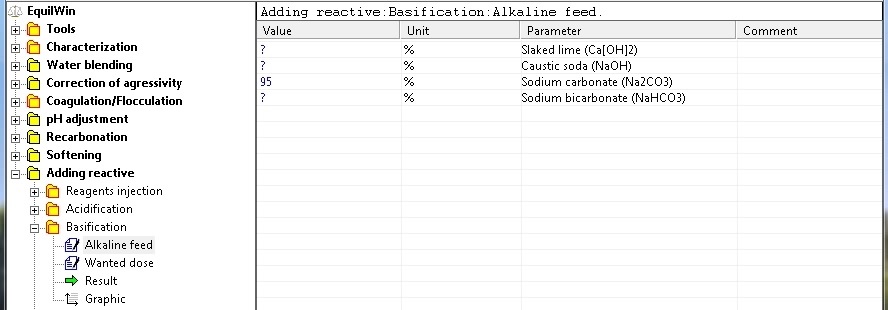
Choice of bases (Alkaline feed)
|
Choice of alkaline reagent and introduction of purity of pure product (in%).
For our example, sodium carbonate with 95% purity as Na2CO3 (commercial product).
|
Wanted dose (Alkaline reagent)
|
Entrance wanted dose of basic example with 45 g of 95% as Na2CO3 per m3 of water (recall the purity Comment)..
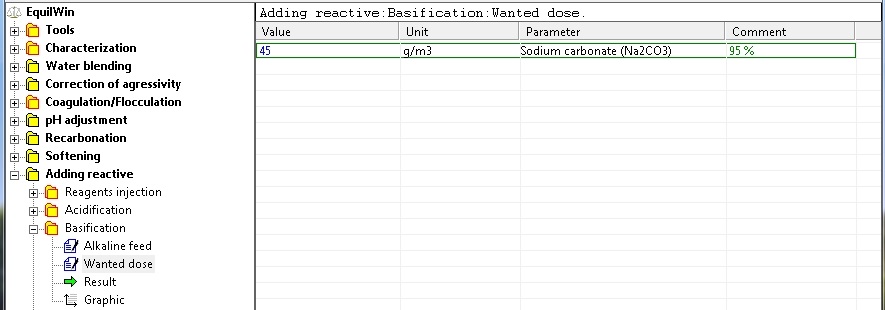
Display results for example of water to be treated:
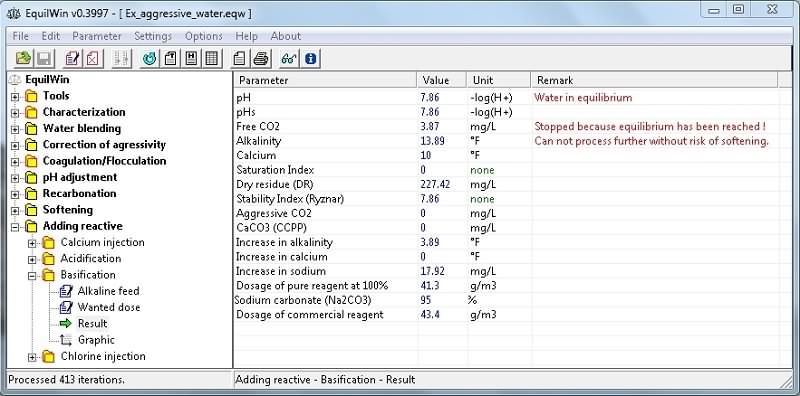
Note: the complete lower aggressive CO2 and CCPP with automatic stop calculations because water is in equilibrium, and therefore the rate of tested treatment can not be achieved (adequate levels are shown).
Also, note increase in sodium ions with Na2CO3 choice (sodium carbonate).
|
Injection of chlorinated reactants
:(Chlorine injection)
|
Used to test the variation of water parameters per action of an addition of chlorinated reagents, therefore in particular variations of pH, alkalinity (TAC)
and aggressive CO2 (among other parameters).
Two options for each entry with known concentrations of reagents):
-
injections of
concentrated
chlorinated reagents (Cl2 chlorine gas and bleaches)
-
injections of diluted chlorine reagents (bleaches)
|
Reactive
(concentrated reagent)
|
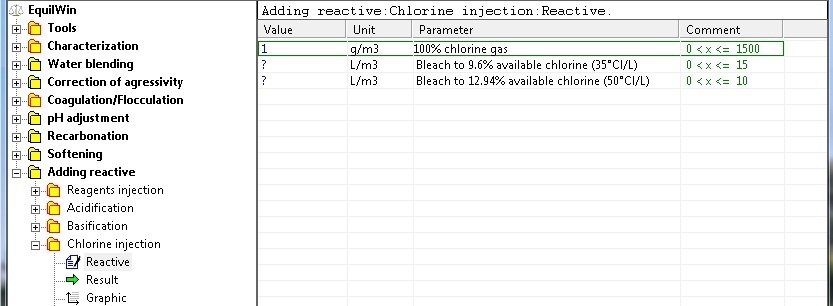
Choice of chlorinated reagents and proposed introduction of value of dose in grams per m3 of water per liter (s) per m3 of water, depending on the product.
For our example, a dose of 1 g/m3 of chlorine gas at 100% purity as Cl2 (chlorine gas).
Note: the input limits have been deliberately set for each reagent.
Display results for the example of water to be treated:
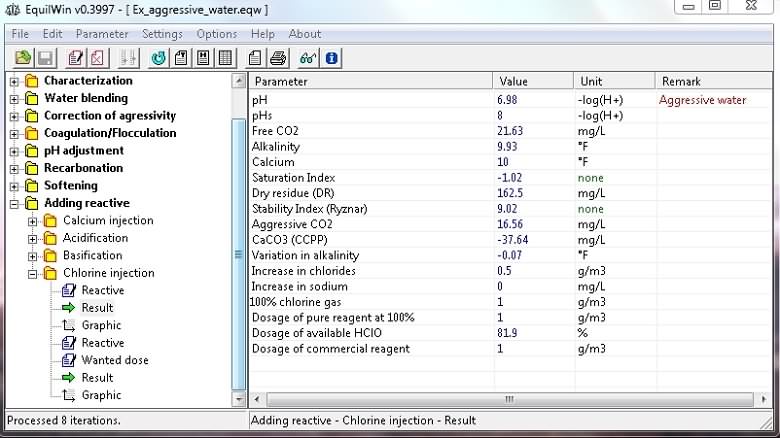
Note the increase of certain ions (chloride and / or sodium), and also an indication of the value of assets in HClO% pure reagent (fraction of the dose of pure reagent).
HClO hypochlorous acid is actually disinfectant active agent. It is designated by the term "Dosage of available HClO" as opposed to free chlorine in reserve in the form of hypochlorite ClO-and combined chlorine.
|
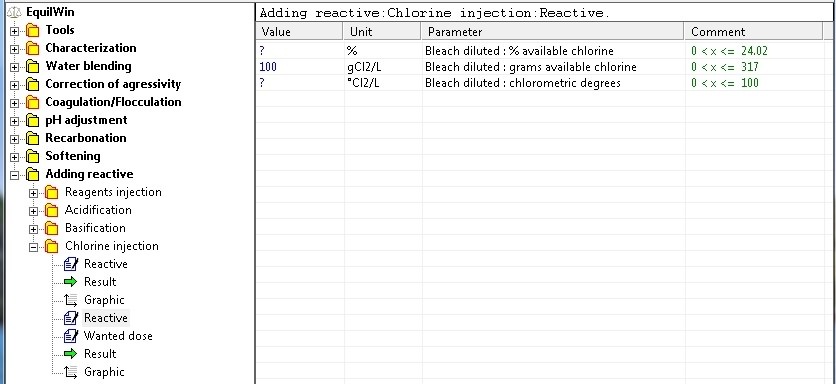
Reactive
(choice of diluted chlorine reagent )
|
Choice of chlorinated reagents and introduction of value of purity (concentration of active ingredient), and therefore % active as Cl2, in grams per liter of solution, or chlorometric degrees (mainly used in France and some countries).
>For our example: choices of diluted bleach to 100 g active CL2 / liter.
Note: the input limits have been deliberately set for each reagent.
|
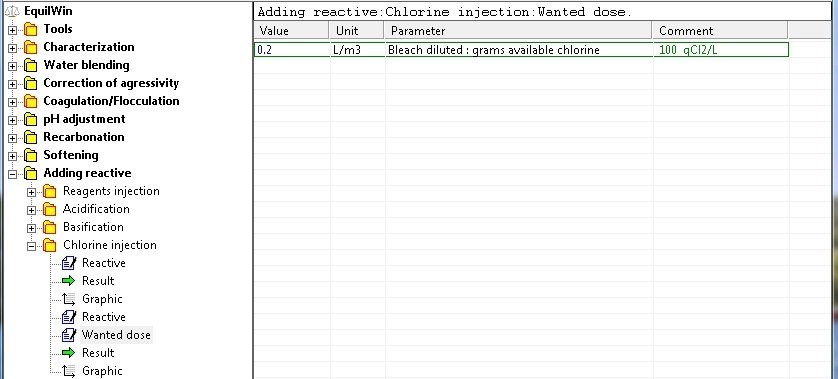
Wanted
dose
(diluted réactive)
|
Enter the desired amount of reagent selected: example 0.2 liters per m3 of water (recall choice and purity Comment)
|
Result
(diluted reactive)
|
Display results for the example of water to be treated:
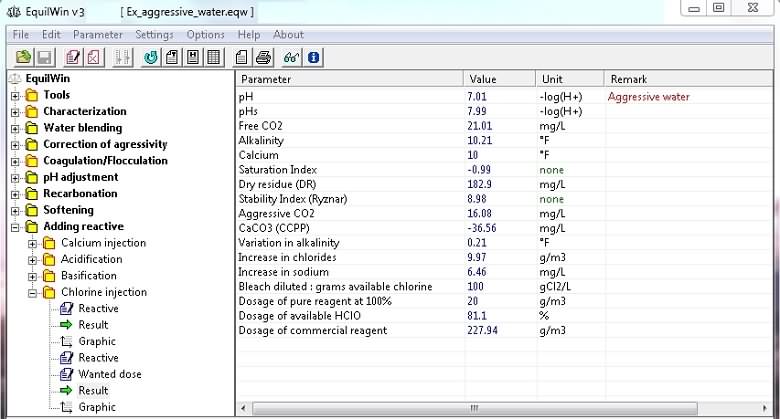
Note the increase of certain ions (chloride and / or sodium), and also an indication of the value of assets in HClO% pure reagent (fraction of the dose of pure reagent).
Note: HClO hypochlorous acid is disinfectant active agent actually .
It is designated by the term
"Dosage of available HClO"
as opposed to free chlorine in reserve in the form of hypochlorite ClO-and combined chlorine.
------------------------
EquilWin
> Software created by Pierre Ravarini.
.


































































